Background
The ectodermal dysplasias are a large, heterogeneous group of inherited disorders that are defined by primary defects in the development of two or more tissues derived from embryonic ectoderm. The tissues primarily involved are the skin and its appendages (hair follicles, eccrine glands, sebaceous glands, nails) and the teeth. Although Thurnam published the first report of a patient with ectodermal dysplasia in 1848, it was not until 1929 that Weech coined the term ectodermal dysplasia.
The ectodermal dysplasias are congenital, diffuse, and nonprogressive. To date, more than 180 distinct forms of ectodermal dysplasia have been described on the basis of phenotype or inheritance pattern. [1] The most common ectodermal dysplasias are X-linked recessive hypohidrotic ectodermal dysplasia (XL-HED or Christ-Siemens-Touraine syndrome; see the image below) and hidrotic ectodermal dysplasia (Clouston syndrome).
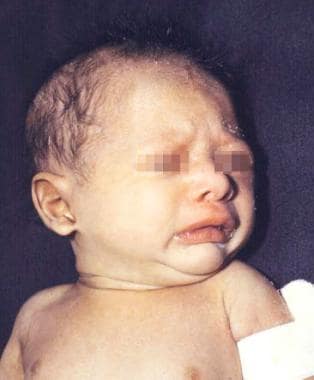 Newborn boy with anhidrotic/hypohidrotic ectodermal dysplasia syndrome showing generalized fine scaling and history of intermittent fever.
Newborn boy with anhidrotic/hypohidrotic ectodermal dysplasia syndrome showing generalized fine scaling and history of intermittent fever.
Classification of ectodermal dysplasias has generally been based on clinical features. Pure ectodermal dysplasias are manifested by defects in ectodermal structures alone, whereas ectodermal dysplasia syndromes are defined by the combination of ectodermal defects in association with other anomalies. However, research has been tending in the direction of classification based on genetic factors. [1, 2] (See Presentation, Classification.)
Several ectodermal dysplasia syndromes may manifest in association with midfacial defects (mainly cleft lip, cleft palate, or both). The following are the three most commonly recognized entities, all of which are caused by mutations in the TP63 gene:
-
Ectrodactyly–ectodermal defects–cleft lip/palate (EEC) syndrome [3]
-
Hay-Wells syndrome, also referred to as ankyloblepharon–ectodermal dysplasia–cleft lip/palate (AEC) syndrome
-
Rapp-Hodgkin syndrome
The care of affected patients depends on which ectodermal structures are involved. (See Treatment.) No specific pharmacologic treatment is available. If cleft lip or palate is present, early repair may lessen facial deformities and improve speech; other midfacial defects or hand/foot deformities may be surgically corrected so as to improve function and reduce physical disfigurement.
Pathophysiology
Ectodermal dysplasia results from the abnormal morphogenesis of cutaneous and/or oral embryonal ectoderm (ie, hair, nails, teeth, eccrine glands). In some forms, mesodermal abnormalities are also present. Characteristic features include the following:
-
Hair defects - A reduction in the number of hair follicles in conjunction with structural hair shaft abnormalities may be seen; structural hair shaft abnormalities may result from aberrations in hair bulb formation and include longitudinal grooving, hair shaft torsion, and cuticle ruffling; hair bulbs may be distorted, bifid, or small [4]
-
Eccrine defects - Eccrine sweat glands may be absent or sparse and rudimentary, particularly in patients with hypohidrotic ectodermal dysplasia [4]
-
Other secretory gland defects - Hypoplasia of the salivary, sebaceous, and lacrimal glands may occur; in some patients, mucous glands may be absent in the upper respiratory tract and in the bronchi, esophagus, and duodenum
-
Dental defects - Abnormal morphogenesis or absence of teeth, as well as enamel defects, may occur [5]
-
Nail dystrophy - Abnormal nail plate formation may result in brittle, thin, ridged, or grossly deformed nails
Although some ectodermal dysplasia syndromes have no known genetic etiology, the number of ectodermal dysplasia syndromes with an identifiable genetic basis is increasing. A 2022 update from the 8th International Conference on Ectodermal Dysplasias listed 49 currently known ectodermal dysplasias for which the molecular genetic basis has been clarified. [1]
Key transcription factors and intracellular signaling pathways that have been implicated in the ectodermal dysplasias include the following [6] :
-
Tumor necrosis factor (TNF)-like/TNV receptor signaling pathway, which involves ectodysplasin A (EDA), the EDA receptor (EDAR), and the EDAR-associated death domain (EDARADD)
-
Wingless related integration site (WNT) signaling pathway
-
Nuclear factor (NF)-κB signaling pathway, which involves the NF-κB essential modulator (NEMO)
-
Transcription factor p63
Etiology
Ectodermal dysplasia results from the abnormal development of embryonic ectodermal structures. The genetic defects responsible for nearly 50 of the ectodermal dysplasias have been identified. [1] However, there remains a need for a more detailed understanding of the pathophysiology underlying most forms of ectodermal dysplasia, particularly with regard to the mechanisms by which the underlying genetic defects impact the growth and development of ectodermal structures.
XL-HED (Christ-Siemens-Touraine syndrome) is caused by mutations in EDA, which encodes the protein EDA, a soluble ligand that activates the NF-κB and JNK/c-fos/c-jun signaling pathways. [7] EDA is important in promoting cell survival, growth, and differentiation. With the use of specialized techniques (including confocal imaging, phototrichogram analysis, and pilocarpineiontophoresis), a complete absence of eccrine ducts, a reduction in hair follicle units and hair follicle density, and a decreased growth rate of terminal hairs have been demonstrated in patients with XL-HED. [8]
Autosomal dominant and autosomal recessive hypohidrotic ectodermal dysplasia are caused by mutations in DL, which encodes EDAR (ie, the EDA receptor). [9] Autosomal recessive hypohidrotic ectodermal dysplasia may also result from mutations in EDARADD, which encodes a protein that interacts with EDAR. A heterozygous mutation in TRAF6 has been described in a patient with hypohidrotic ectodermal dysplasia. [10]
Hidrotic ectodermal dysplasia (Clouston syndrome), which is an autosomal dominant disorder, is caused by mutations in GJB6, which encodes connexin 30, a component of intercellular gap junctions. [11]
Ectodermal dysplasia with immunodeficiency (EDA-ID) and EDA-ID with osteopetrosis and lymphedema (OL-EDA-ID) are both caused by mutations in NEMO, which encodes NEMO, the regulatory subunit of the inhibitor-kappa kinase complex that regulates NF-κB activity. [12, 13, 14]
AEC (Hay-Wells) syndrome, Rapp-Hodgkin syndrome, EEC syndrome, limb-mammary syndrome, split hand-split foot malformation syndrome, and acro-dermato-ungual-lacrimal-tooth (ADULT) syndrome are all caused by mutations in TP63, which encodes the transcription factor p63. [15, 16]
The following are selected examples of genetic defects known to underlie other ectodermal dysplasias:
-
Keratitis-ichthyosis-deafness (KID) syndrome - Mutations in GJB2, which encodes connexin 26 [17]
-
Margarita Island ectodermal dysplasia - Mutations in PVRL1, which encodes nectin-1 [18]
-
Ectodermal dysplasia with skin fragility - Mutations in PKP1, which encodes plakophilin 1 [19]
-
Goltz syndrome (focal dermal hypoplasia) - Mutations in PORCN [20]
-
Naegeli-Franceschetti-Jadassohn syndrome and dermatopathia pigmentosa reticularis - Mutations ie KRT14, which encodes keratin 14 [21]
-
Pachyonychia congenita type I - Mutations in either KRT6A (keratin 6a) or KRT16 (keratin 16)
-
Incontinentia pigmenti - Mutations in NEMO [31]
-
Anhidrotic ectodermal dysplasia with common variable immunodeficiency - Mutations in ORAI1, which encodes oral calcium release-activate calcium modulator 1 [32]
Epidemiology
US and international statistics
The frequency of the different ectodermal dysplasias in a given US population is highly variable. The prevalence of HED, the most common variant, has been estimated to be one case per 100,000 births.
The worldwide prevalence of ectodermal dysplasia has been estimated at seven cases per 10,000 births.
Age-, sex-, and race-related demographics
Clinical recognition of ectodermal dysplasia may occur anywhere from birth to childhood, depending on symptom severity and the presence of associated complications. Many patients are not diagnosed until infancy or childhood, when dental anomalies, nail abnormalities, or alopecia become apparent.
AEC (Hay-Wells) syndrome may manifest at birth as ankyloblepharon in association chronic scalp erosions. HED may manifest as scaling and erythema at birth. EEC syndrome and other related ectrodactyly syndromes (eg, ADULT syndrome and limb-mammary syndrome) are usually recognized at birth as a result of the characteristic limb deformities. Patients with anhidrosis or hypohidrosis may present in early infancy with recurrent episodes of hyperpyrexia.
X-linked recessive HED is fully expressed only in males. Female carriers outnumber affected men, but females show little or no signs of the condition. X-linked recessive anhidrotic EDA-ID and the X-linked recessive syndrome of OL-EDA-ID are also seen exclusively in males. The remaining ectodermal dysplasias have no sexual predilection.
The ectodermal dysplasias have been reported most often in Whites, but they have also been observed in persons of other races. Hidrotic ectodermal dysplasia has been reported in an extensive kindred of French-Canadian origin.
Prognosis
For most patients with ectodermal dysplasia, the prognosis is very good. Morbidity and mortality are related to the absence or dysfunction of eccrine and mucous glands. Beyond early childhood, life expectancy ranges from normal to slightly reduced.
If hypohidrosis is recognized in the neonatal period and managed appropriately, the lifespan for a person diagnosed with one of the common types of ectodermal dysplasia has not been shown to be shorter than average. Intermittent hyperpyrexia may occur in infants with decreased sweating. Mortality approaches 30%. Recurrent high fever may also lead to seizures and neurological sequelae.
Pharyngitis, rhinitis, cheilitis, and dysphagia may result from reduced numbers of functional mucous glands in the respiratory and gastrointestinal tracts.
Growth failure is common. [37]
In patients with AEC (Hay-Wells) syndrome or Rapp-Hodgkin syndrome, severe inflammatory scalp dermatitis with erosions may result in frequent infections and cause scarring alopecia.
In some rare types of ectodermal dysplasia, lifespan can be affected. For example, patients with EDA-ID are at risk for significant morbidity and mortality related to recurrent infections and failure to thrive.
Patient Education
It is important to provide early guidance about temperature regulation, acceptable activities, and the risk of hyperpyrexia from febrile illnesses. Patients and families should be informed that antipyretics are not effective in treating hyperpyrexia associated with hypohidrosis. Caregivers should be instructed regarding proper skin care and the need to monitor for signs of infection in patients with chronic scalp dermatitis and erosions.
Additional information and support for families is available through the National Foundation for Ectodermal Dysplasias.
-
Newborn boy with anhidrotic/hypohidrotic ectodermal dysplasia syndrome showing generalized fine scaling and history of intermittent fever.
-
Wrinkled, hyperpigmented skin around eyes and everted lips are typical characteristics of anhidrotic/hypohidrotic ectodermal dysplasia syndrome.
-
Typical cleft lip/palate and maxillary hyperplasia in patient with Rapp-Hodgkin syndrome.
-
Abnormal hair shaft showing pili torti and longitudinal groove (pili canaliculi) from patient with Rapp-Hodgkin syndrome.
-
Hands of father and son with Rapp-Hodgkin syndrome. Nails have same characteristics; they are brittle, thin, and dystrophic.
-
Ectodermal dysplasia-ectrodactyly-clefting syndrome. Light-colored hair and scalp and earlobe defects are observed. Cleft lip and palate result in characteristic nasal contour.
-
Ectrodactyly observed in individual with ectodermal dysplasia-ectrodactyly-clefting syndrome.







