Practice Essentials
Lichen planus (LP; see the image below) is a cell-mediated immune response of unknown origin. It may be found with other diseases of altered immunity, such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosus, and myasthenia gravis. A rare type of LP, familial bullous lichen planus, could be gene-related. LP may be triggered by diuretics and antimalarials, metal fillings (causing oral LP), stress, and infection. It has been found to be associated with hepatitis C virus (HCV) infection.
Signs and symptoms
The following may be noted in the patient history:
-
Lesions initially developing on flexural surfaces of the limbs, with a generalized eruption developing after a week or more and maximal spreading within 2-16 weeks
-
Pruritus of varying severity, depending on the type of lesion and the extent of involvement
-
Oral lesions that may be asymptomatic, burning, or even painful
-
In cutaneous disease, lesions typically resolve within 6 months (>50%) to 18 months (85%); chronic disease is more likely with oral LP or with large, annular, hypertrophic lesions and mucous membrane involvement
In addition to the widespread cutaneous eruption, LP can involve the following structures:
-
Mucous membranes
-
Genitalia
-
Nails
-
Scalp
The clinical presentation of LP has several variations, as follows:
-
Hypertrophic LP
-
Atrophic LP
-
Erosive/ulcerative LP
-
Follicular LP (lichen planopilaris)
-
Annular LP
-
Linear LP
-
Vesicular and bullous LP
-
Actinic LP
-
LP pigmentosus
-
LP pemphigoides
See Clinical Presentation for more detail.
Diagnosis
Direct immunofluorescence study reveals globular deposits of immunoglobulin M (IgM) and complement mixed with apoptotic keratinocytes. No imaging studies are necessary.
Distinguishing histopathologic features of LP include the following:
-
Hyperkeratotic epidermis with irregular acanthosis and focal thickening in the granular layer
-
Degenerative keratinocytes (colloid or Civatte bodies) in the lower epidermis; in addition to apoptotic keratinocytes, colloid bodies are composed of globular deposits of IgM (occasionally immunoglobulin G [IgG] or immunoglobulin A [IgA]) and complement
-
Linear or shaggy deposits of fibrin and fibrinogen in the basement membrane zone
-
In the upper dermis, a bandlike infiltrate of lymphocytic (primarily helper T) and histiocytic cells with many Langerhans cells
See Workup for more detail.
Management
LP is a self-limited disease that usually resolves over several months but can sometimes take years to do so. It recurs in about 20% of patients, and it may linger for years, particularly oral LP. LP commonly results in postinflammatory hyperpigmentation and occasionally leaves hypertrophic scars, which may be the result of scratching. Mild cases can be treated with fluorinated topical steroids. More severe cases, especially those with scalp, nail, and mucous membrane involvement, may necessitate more intensive therapy. Classic idiopathic LP characteristically is highly sensitive to corticosteroid treatment.
Pharmacologic therapies include the following:
-
LP of the oral mucosa - Topical steroids; topical calcineurin inhibitors (eg, tacrolimus, pimecrolimus); oral or topical retinoids (with close monitoring of lipid levels [6] )
Patients with widespread LP may respond to the following:
See Treatment and Medication for more detail.
Background
Lichen planus (LP) is a pruritic eruption that can be associated with hepatitis C. Lesions are characteristically papular, purple or violaceous in color, polygonal, and often peripherally located on the distal extremities. LP has various distinct subtypes and clinical presentations, including but not limited to those affecting the genitalia or mucous membranes. Although the pathophysiology is unclear, it is an immunologically mediated reaction.
Pathophysiology
LP is a cell-mediated immune response of unknown origin. It may be found with other diseases of altered immunity; such conditions include ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosus, and myasthenia gravis.
Associations have been noted between LP and hepatitis C virus (HCV) infection, [8, 9, 10, 11, 12, 13] chronic active hepatitis, and primary biliary cirrhosis. [14] In one meta-analysis, 16% of patients with LP had hepatitis C infection. [10] This association has been shown to exist in all regions of the world, including North America. [11] A workup for hepatitis C should be considered in patients with widespread or unusual presentations of LP. Onset or exacerbation of LP has also been linked to stressful events. [15]
Etiology
The exact cause of LP has not been established, though the condition is known to be immunologically mediated. The initiating antigen is unclear; however, Langerhans cells process the antigen to T lymphocytes, resulting in an epidermotropic infiltrate. Histologically, the inflammation is described as a lichenoid infiltrate, effacing the dermoepidermal junction.
Some patients with LP have a positive family history. [16] It has been noted that affected families have an increased frequency of human leukocyte antigen (HLA)-B7. Others have found an association between idiopathic LP and HLA-DR1 and HLA-DR10; thus, LP may be influenced by a genetic predisposition.
Epidemiology
US and international statistics
In the United States, LP is reported in approximately 1% of all new patients seen at health care clinics. Some areas have reported a higher incidence in December and January.
Internationally, no significant geographical variation in frequency exists for LP.
Age-, sex-, and race-related demographics
More than two thirds of LP patients are between the ages of 30 and 60 years; however, LP can occur at any age. [17]
No significant differences in the incidence of LP are noted between male and female patients; however, in women, LP may present as desquamative inflammatory vaginitis. [18]
No racial predispositions have been noted for LP.
Prognosis
The prognosis for LP is good, in that most cases regress within 18 months. Some cases recur. In LP, atrophy and scarring are seen in hypertrophic lesions and in lesions on the scalp. Cutaneous LP does not carry a risk of skin cancer, but ulcerative lesions in the mouth, particularly in men, do occasionally exhibit malignant transformation; however, the rate of malignant transformation for oral LP is low (< 2% in one report). [19] Vulvar lesions in women may also be associated with squamous cell carcinoma.
Patient Education
Patients should be informed about the self-limiting nature of LP. Because LP is not common, no large randomized controlled clinical trials have been conducted for therapy. It may be necessary to try several different treatments. Patients should also be told about the small likelihood of recurrence and the potential adverse effects from the various treatments offered.
-
Lichen planus on flexor part of wrist.
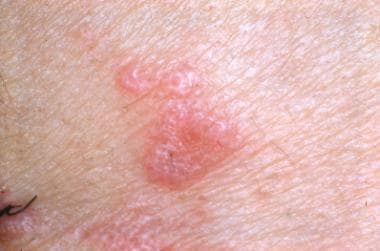
-
Close-up view of lichen planus.
-
Lichen planus shows Wickham striae (white, fine, reticular lines).
-
Lichen planus on oral mucosa with ulceration in center of lesion appears with whitish papules and plaques in periphery.
-
Lichen planus lesion. Image from Syed Wali Peeran, with no alterations, Wikimedia Commons (https://commons.wikimedia.org/wiki/File:Lichen_planus-Skin_lesion.jpg).
-
Intraoral lichen planus lesion. Image from Syed Wali Peeran, with no alterations, Wikimedia Commons (https://commons.wikimedia.org/wiki/File:Lichen_planus-intra_oral_lesion.jpg).
-
Bandlike infiltrate of lymphocytes obscures dermoepidermal junction. Eosinophilic apoptotic keratinocytes (colloid bodies) are visible. Image from Clay Cockerell, MD, Cockerell Dermatopathology, Dallas, TX.
-
Higher-power view. Wedge-shaped hypergranulosis and individual degenerated keratinocytes are visible in addition to hyperkeratosis. Vacuolar degeneration is visible. Image from Clay Cockerell, MD, Cockerell Dermatopathology, Dallas, TX.