Background
Porphyria cutanea tarda (PCT) is a term encompassing a group of acquired and familial disorders in which activity of the heme synthetic enzyme uroporphyrinogen decarboxylase (UROD) is deficient. [1] It is known by many other names as well, including symptomatic porphyria, idiosyncratic porphyria, chemical porphyria, and acquired hepatic porphyria. [2] Approximately 80% of all cases of PCT are acquired and 20% familial, though the ratio may vary in different geographic regions and ethnic groups.
Familial PCT most often arises from autosomal dominant inheritance of a single mutation of the gene UROD. Human UROD has been mapped to band 1p34. To date, more than 140 UROD mutations have been listed in the Human Genome Mutation Database. A rare recessive familial type of PCT in which both UROD alleles are mutated is termed hepatoerythropoietic porphyria. [3] Familial PCT without detectable UROD mutations has been reported. [4]
The common acquired form, sporadic PCT, occurs in individuals whose UROD DNA sequences are normal, but who may have other genetically determined susceptibilities to inhibition of UROD activity. Acquired porphyria in large populations exposed to polyhalogenated aromatic hydrocarbon hepatotoxins has been referred to as epidemic PCT. Hepatic tumors producing excess porphyrins are rare causes of PCT-like disorders.
Clinical expression of both sporadic and familial PCT most often requires exposure to environmental or infectious agents or the presence of coexisting conditions that adversely affect hepatocytes and result in hepatic siderosis. Ethanol intake, estrogen therapy, hemochromatosis genes, hepatitis, and HIV infection are among these contributory factors. The increased oxidative stress associated with all of these factors has been shown to reduce hepatic expression of the gene encoding hepcidin, a regulator of iron absorption and metabolism, thereby increasing iron absorption and iron overload. [5]
Excess iron facilitates formation of toxic oxygen species, thus amplifying porphyrinogenesis by catalyzing formation of oxidative inhibitors of UROD enzyme activity. [6] Accumulating porphyrins in hepatocytes may then further downregulate hepcidin gene expression. [5] Most patients with PCT have an increased iron burden; iron-reduction therapies can lead to clinical and biochemical remissions, and subsequent reaccumulation of iron stores may lead to symptomatic recurrence. [7, 8, 9, 10, 11, 12]
Reduced UROD activity causes polycarboxylated porphyrinogen intermediaries of heme synthesis to accumulate in hepatocytes; these excess substrates then undergo iron-facilitated spontaneous oxidization to photoactive porphyrins. Porphyrin by-products of the pathway exit the hepatocytes, are distributed throughout the body in blood plasma, mediate photo-oxidative chemical reactions causing skin lesions, and yield the abnormal excretory porphyrin profiles that characterize PCT. Partial oxidation of uroporphyrinogen to the UROD inhibitor uroporphomethene occurs in murine PCT models and has been suggested as a pathogenic mechanism in the human disease. [13]
Management includes elimination or modification of contributory factors (see above), photoprotection, and specific treatments (eg, low-dose antimalarials and phlebotomy). [14, 15]
Pathophysiology
When hepatic UROD activity falls below the critical threshold, porphyrin by-products of the heme biosynthetic pathway with 4-8 carboxyl group substituents are overproduced. These porphyrins are reddish pigments that accumulate in the liver and are disseminated in plasma to other organs. Porphyrins with high carboxyl group numbers are water-soluble and excreted primarily by renal mechanisms. The 8-carboxyl porphyrin is termed uroporphyrin; 4-carboxyl porphyrins include coproporphyrin and isocoproporphyrin, which are chiefly excreted in feces.
Reduction of hepatic UROD activity to approximately 25% of normal, most often reflecting effects of multiple genetic or exogenous inhibitory factors, is required for clinical disease expression. Symptomatic disease occurs more often in patients with a genetic predisposition to PCT, in that fewer external or exogenous factors are needed to decrease UROD activity. [2]
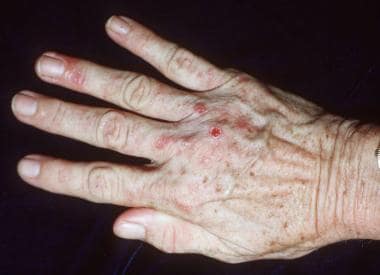
Porphyrins are photoactive molecules that efficiently absorb energy in the visible violet spectrum. Photoexcited porphyrins in the skin mediate oxidative damage to biomolecular targets, causing cutaneous lesions. The most common photocutaneous manifestations of PCT are due to increased mechanical fragility after sunlight exposure; erosions and blisters form painful indolent sores that heal with milia, dyspigmentation, and scarring (see the image below).
Other common features of PCT include hypertrichosis, sclerodermalike plaques that may develop dystrophic calcification, and excretion of discolored urine that resembles port wine or tea, which is due to the presence of the porphyrin pigments.
Etiology
The unifying underlying cause of all forms of PCT is reduction of UROD activity to a critical point during hepatic heme synthesis. Genetic, environmental, or infectious contributory or susceptibility factors, acting singly or (more often) in concert, [16, 17, 18] inhibit UROD activity to that critical point of insufficiency, resulting in the onset of clinical symptoms.
Alcohol effects on hepatocytes may precipitate PCT by making stored hepatic iron more available for catalyzing oxidation reactions, by generation of reactive oxygen species, or by induction of hepatic cytochromes, all of which may facilitate oxidation of uroporphyrinogen to UROD inhibitors. [19]
The role of estrogen in inhibition of UROD activity is not firmly established, but it may be similar to that of alcohol in generating toxic oxygen or inducing cytochromes, thus potentiating uroporphyrinogen oxidation.
A strong association between PCT and hereditary hemochromatosis genes causing hepatic siderosis has been established. [16, 20, 21, 22]
Hepatitis virus infections are frequently associated with PCT. [20, 23, 24] Hepatitis C virus (HCV) infection occurs at a rate of greater than 50% in populations studied in several European countries and in the United States, whereas in other regions, the concordance is less frequent. [25] Increased hepatic iron often is found in patients with hepatitis C. [26, 27]
It was previously believed that coexistent HIV could exacerbate PCT. However, it is now believed that the exacerbation of PCT with contraction of HIV results from coinfection with HCV. [28]
Tobacco smoking is a behavioral characteristic frequently observed among patients with PCT. [16, 18] Smoking has been associated with earlier onset of symptoms in sporadic PCT, with a putative mechanism involving induction of an hepatic cytochrome that may contribute to oxidation of uroporphyrinogen, [29] but evidence for smoking as an independent pathogenic factor is not yet robust.
Environmental exposure to aromatic polyhalogenated hepatotoxins also induces hepatic cytochromes, thus potentiating production of oxidation products capable of inhibiting UROD activity.
Epidemiology
US and international statistics
PCT is the most common porphyria, accounting for 80-90% of all porphyrias seen in clinical practice. [30] A registry was established by the National Institutes of Health (NIH)-funded Porphyrias Consortium (https://pc.rarediseasesnetwork.org/) to enable more accurate enumeration of cases of various types of porphyria occurring in the US population. Estimates of the frequency of PCT have varied. A 2017 study cited an estimate of one case per 25,000 in the United States. [28] The Genetic and Rare Diseases Information Center (GARD) stated that fewer than 50,000 people in the United States have PCT. [31]
PCT has a prevalence of about 40 new diagnoses per 1 million people per year. [32] Higher prevalence figures have been reported among various European populations. A high prevalence of PCT among South African Bantu people has been linked with a propensity for hepatic siderosis. Fractions of studied PCT cases reported as familial have varied widely—for example, 14.6% in Spain, [33] 24% in Denmark, [34] and 50% in Chile. [35]
Age-, sex-, and race-related demographics
Sporadic PCT typically manifests in adulthood. Symptoms of familial PCT typically first appear in adults heterozygous for a UROD gene mutation, but they have also been reported in heterozygous children. [36] When biallelic mutations are present (homozygotes or compound heterozygotes), symptoms may be severe, with onset in early childhood. [37] Milder phenotypes with somewhat later onset have also been observed. [38] PCT-like disorders resulting from exposure of large numbers of people to hepatotoxic chemicals have afflicted people of all ages.
PCT occurs in both sexes. Older reports indicated a great preponderance of PCT in men; subsequent surveys indicated an equal ratio among men and women. [28]
PCT occurs in persons of all ethnic groups. [39]
Prognosis
The major morbidity of PCT is due to skin fragility and blistering, which preclude manual labor and hamper daily activities. The subsequent erosions represent full-thickness epidermal loss; they are painful and often become thickly crusted and secondarily infected. Healing is slow and leaves pigmentary changes, milia, and atrophic scars.
PCT has been associated with the development of hepatocellular carcinoma (HCC), chiefly in populations of older men with long-standing active disease, heavy ethanol intake, and cirrhosis. Most of the studies predate recognition of hepatitis C prevalence in populations with PCT or HCC; many reported cancers may have been, at least in part, sequelae of chronic HCV infection. [40]
Patient Education
Patients should be educated about the role of sunlight in eliciting the skin lesions and about methods of sunlight avoidance. Because porphyrins absorb radiant energy most efficiently at very long ultraviolet (UV) and visible light wavelengths, topical sunscreens must contain ingredients that either scatter or block long UV and visible light rays to offer any practical protection.
Sunscreens with titanium dioxide or zinc oxide are recommended because the porphyrin-inducing UV wavelength is 400-410 nm, and these barrier sunscreens have better protection for this wavelength than sunscreens with chemical blockers. [28] Use of light-exclusive clothing and lifestyle alterations are usually necessary to alleviate photocutaneous reactions until remissions can be achieved.
The need to avoid iron-containing dietary supplements, alcohol, and smoking should be stressed. Dietary iron is not usually a major problem and can be managed with moderation in consumption of red meats, but some patients may benefit from a nutritionist's guidance regarding the iron content of foods. Adequate dietary vitamin C should be consumed.
-
Thickened skin with blisters, scars, and milia. Image from Dirk Elston, MD.
-
Close-up image of blisters, scarring, and milia. Image from Dirk Elston, MD.
-
Subepidermal bulla, festooning of rete ridges, hyalinization of blood-vessel walls, solar elastosis, and caterpillar bodies. Image from Dirk Elston, MD.
-
Fluorescence of urine with Wood light examination. Image from Brooke Army Medical Center Teaching File.