Practice Essentials
A Spitz nevus is a cutaneous lesion that most often arises in childhood as a red, dome-shaped papule, but may rarely occur in adults. Spitz nevi may appear suddenly and grow rapidly. Three classes of Spitz nevi are recognized: typical Spitz nevus, atypical Spitz tumor, and spitzoid melanoma; this classification recognizes the spectrum from benign to malignant behavior of these lesions. [1]
Spitz nevi were first described in 1948 by Sophie Spitz, who termed them juvenile melanoma, to distinguish them from malignant melanomas in adults, because although histologically diagnosed as melanomas, most of these lesions did not show aggressive behavior. [2] Benign Spitz nevi can be recognized by routinely staining, without immunostaining or genomic testing. [3] In contrast, with atypical Spitz tumors in particular, identifying lesions that have metastatic potential has proved a continuing challenge. [4] Even today, no set of criteria can be used to predict the clinical outcome of atypical Spitz tumors with absolute assurance. However, immunohistochemistry and molecular studies have been helpful in differentiating difficult cases, and next‐generation sequencing can improves diagnostic accuracy by identifying genetic drivers.
Pathophysiology
A Spitz nevus can arise de novo or in association with an existing melanocytic nevus. [6] Most Spitz nevi result from the following genetic events\ [3, 5] :
-
Fusions involving tyrosine kinase genes (eg, ROS1, ALK, NTRK1, NTRK3, MET)
-
Fusions involving serine-threonine kinase genes (eg, BRAF)
-
Mutations in HRAS or MAP3K8
Molecular techniques have defined subtypes of Spitz tumors, including benign Spitz nevus, atypical Spitz tumor, and spitzoid melanoma. [7, 8, 9, 10, 11, 12, 13]
The World Health Organization defines atypical Spitz tumor (which it terms Spitz melanocytoma) as a classification of Spitz tumors that shares genetic and morphologic characteristics between Spitz nevi and Spitz melanoma and has variable potential for malignant progression. [14] The essential features of these tumors is as follows:
-
Nevus consistsing of epithelioid and spindled melanocytes and containing morphologic and genetic atypia sufficient to distinguish it from Spitz nevus but insufficient for a diagnosis of melanoma
-
Has a higher risk of sentinel lymph node involvement but typically has an indolent behavior; potential for malignancy is difficult to discern
-
Often contains HRAS mutation or tyrosine kinase fusions involving ROS1, NTRK1, NTRK3, ALK, BRAF, RET, MET and MAP3K8
Epidemiology
Note the following:
-
Frequency – Exact data on the incidence or prevalence are not available. Spitz nevi are estimated to represent less than 1% of all childhood melanocytic nevi.
-
Race – Spitz nevi have been described most frequently in fair-skinned individuals. One study reviewed 130 cases in a Hispanic population, demonstrating that Spitz nevi are not restricted to White patients. [15]
-
Sex – Both sexes are equally affected. Some authors describe a slight female predominance. [16]
-
Age – About 50% of cases occur in children younger than 10 years; 70% of all cases are diagnosed during the first 2 decades of life. Congenital Spitz nevi are also found. [17]
Prognosis
The prognosis is good. Recurrences should be treated with re-excision. These lesions are clinically benign. A 2011 study reporting on 157 patients with Spitz-type melanotic lesions suggests that atypical Spitz tumors pose a minimal threat of mortality but have an increased risk of melanoma and a moderate risk of metastasis to regional nodes. Aggressive treatment is usually not needed, but monitoring for signs of relapse, as well as subsequent melanomas, is recommended. [18] Mitoses and inflammation are indicators of increased aggressiveness. [19] Using single morphologic features to determine prognosis has severe limitations. [20]
History and Physical Examination
Spitz nevi are typically single, dome-shaped, red or pigmented papules or nodules (see the image below). Most occur on the face or legs; rare cases of oral Spitz nevi have also been reported. [21] The color may vary from nonpigmented through pink to orange-red. Some lesions are pigmented, especially those found on lower extremities.
After its appearance, the lesion tends to grow rapidly and may reach a size of 1 cm within 6 months. After the rapid initial growth phase, Spitz nevi tend to become static; however, color changes may be observed. Bleeding and pruritus rarely occur
Misdiagnosis of Spitz nevi as melanomas and misdiagnosis of melanomas as Spitz nevi is a possibility. Clinical features that strengthen the probability that a lesion is a Spitz nevus include young patient age and a well-demarcated and symmetrical lesion, in concert with histologic findings of maturation and dispersion of melanocytes at its base and the presence of epithelial hyperplasia. However, no criterion is absolutely reliable. Histopathologic differentiation from melanomas is equivocal in up to 8% of cases.
Diagnostic Considerations
In addition to melanoma, the differential diagnosis of Spitz nevi includes the following:
-
Epidermal nevus
-
Granulomas
-
Histiocytoma (eg, juvenile xanthogranuloma)
-
Vascular tumors
-
Other melanocytic nevi
Workup
On dermatoscopy, Spitz nevi show a starburst pattern (ie, pigmented streaks symmetrically distributed at the periphery of the lesion). [22] A 2015 multicenter study found that Spiz nevi show a multicomponent and nonspecific pattern. [23]
Histologic findings
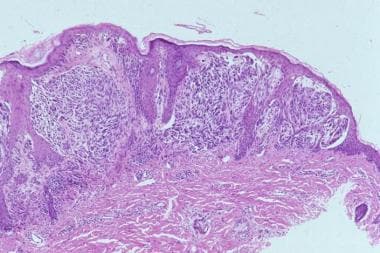
Most Spitz nevi are predominantly compound, although junctional and intradermal lesions are also observed. The sine qua non of the diagnosis is the presence of large and/or spindle-shaped melanocytes, usually in nests. The nests are composed of an admixture of spindle cells and/or epithelioid cells, although frequently, the spindle-shaped cells predominate (see the images below).
The spindle cells are usually observed in a fascicular arrangement, and typically have a vertical orientation. These cells have abundant cytoplasm and contain a vesicular nucleus with a conspicuous nucleolus. The epithelioid cells have prominent nucleoli. Tumors composed only of epithelioid cells, those that lack dispersion at the base, those that are grossly asymmetrical, those with deep mitoses, and those in older individuals are more likely to represent malignant melanoma. [24]
Striking symmetry, sharp lateral demarcation, absent (or rare) mitoses, absence of atypical mitoses, presence of eosinophilic and periodic acid-Schiff (PAS)–positive globules (Kamino bodies) and nondisruptive (single-file like) infiltration of collagen are important features indicating the diagnosis of Spitz nevi. Single-file melanocytes may also be observed in the reticular dermis located at the base of the lesion (dispersion).
Another important feature is the maturation of cellular elements toward the dermis. Pagetoid spread of the melanocytes is usually confined to the center of the lesion; when present, it can cause confusion with melanoma.
The epidermis is hyperkeratotic and acanthotic. A cleavage artifact of fixation is commonly noticed above the nests and around superficial dermal elements. Cytologic features, such as oval vesicular nuclei with a single prominent nucleolus, help to differentiate Spitz nevi from melanoma. [25]
The histologic distinction between Spitz nevi and melanomas is equivocal in many cases, and pathologists often seek a second opinion. [26]
Histopathologic evaluation is indicated. The value of including individual proliferation index and histopathologic parameters (eg, Ki-67, Pi-21, fatty acid synthetase) into models of predictive probabilities is uncertain, but a panel of markers including Ki-67, HMB-45, and S100A6 can be helpful in establishing a histologic diagnosis. In benign Spitz nevi, Ki-67 staining is usually absent in dermal nuclei, HMB-45 staining demonstrates a gradient, and S100A6 diffusely stains the lesion. Melanomas show the opposite patterns. [27]
Mass spectrometry using a proteomic signature may help differentiate benign Spitz nevi from spitzoid melanomas. [28]
Genetic analysis
Identification of driver genetic alterations is the only reliable way to distinguish morphologically atypical Spitz neoplasms from conventional melanomas with spitzoid features. [5]
Analyses of mutations of BRAF, NRAS, and HRAS were promising in distinguishing Spitz nevi from melanomas, but BRAF mutations do not separate all Spitz nevi from spitzoid melanomas and other melanocytic proliferations. [29, 30, 31] TERT promoter mutations are common in spitzoid lesions with an aggressive course, but some similar lesions with more indolent behavior have been described. [32] Mutations in receptor tyrosine kinases ALK, ROS1, NTRK1, and RET have been identified, and oncogenic fusions in TRK family receptor tyrosine kinases, including NTRK3, have been found in Spitz tumors. [33]
Immunohistochemistry and comparative genomic hybridization have proved helpful. [34] As specific genes related to pathogenesis and behavior are identified, next-generation sequencing and proteomics will be used increasingly to clarify the diagnosis of atypical Spitz tumors. Proteomics may be particularly important, as gene silencing may be as important as gene loss. [28]
A rare variant is the desmoplastic Spitz nevus in which the proliferating large epithelioid and/or fusiform melanocytes are embedded in a desmoplastic stroma with thick eosinophilic collagen bundles. [35] Spitz nevi showing architectural features of Clark nevi (dysplastic nevi) were also described in a small series and have been termed Spark nevi or spastic nevi. The spindle cells were oriented parallel to the epidermis with fused rete and lamellar fibroplasia. [36]
A case of a pseudogranulomatous variant (with inflammatory infiltrate mimicking a granulomatous dermatitis) has beendescribed. [37]
Treatment
Surgical excision (generally with local anesthesia) of lesions suspected of being Spitz nevi is recommended. Consult a plastic surgeon or head and neck surgeon if the excision requires extensive repair.
Histopathologic evaluation of the margins of the specimen is indicated. [38, 39] Sentinel lymph node biopsy is not justified for surgical staging. [40] However, a series of 12 cases with atypical Spitz nevi showed nodal micrometastases in one third of the patients, suggesting a not-yet understood metastatic potential.
Regular follow-up, preferably by a dermatologist, is indicated. Patients should be educated about sun protection and self-examination of the skin.
-
Spitz nevus on the ear of a child.
-
Microphotograph (low power).
-
Microphotograph (medium power).
-
Microphotograph (low power).
-
Microphotograph (medium power).