Background
Legionnaires disease (LD) is the pneumonia caused by Legionella pneumophila. Legionnaires disease also refers to a more benign, self-limited, acute febrile illness known as Pontiac fever, which has been linked serologically to L pneumophila, although it presents without pneumonia. Pontiac fever usually is self-limiting and typically does not require antibiotics administration. [1, 2, 3, 4]
L pneumophila is an important cause of nosocomial and community-acquired pneumonia (CAP) and must be considered a possible causative pathogen in any patient who presents with atypical pneumonia. Empiric antibiotic coverage for CAP usually includes classes of antibiotics that have Legionella coverage such as fluroquinolones, macrolides and tetracyclines.
The Legionella bacterium first was identified in the summer of 1976 during the 58th annual convention of the American Legion, which was held at the Bellevue-Stratford Hotel in Philadelphia. Infection was presumed to be spread by contamination of the water in the hotel's air conditioning system. The presentation ranged from mild flulike symptoms to multisystem organ failure. Of the 182 people infected, 29 died.
Although Legionella was not identified until 1976, L pneumophila subsequently was found in a clinical specimen dating to 1943 and, according to retrospective analysis, may have been responsible for pre-1976 pneumonia epidemics in Philadelphia; Washington, DC; and Minnesota.
Legionnaires disease is the term that collectively describes infections caused by members of the Legionellaceae family.
Bacterial characteristics
The Legionella bacterium is a small, aerobic, waterborne, gram-negative, unencapsulated bacillus that is nonmotile, catalase-positive, and weakly oxidase-positive. It is a fastidious organism and does not grow anaerobically or on standard media. Buffered charcoal yeast extract (CYE) agar is the primary medium used for isolation of the bacterium. [1, 2, 3, 4]
The Legionellaceae family consists of more than 42 species, constituting 64 serogroups. L pneumophila is the most common species, causing up to 90% of the cases of Legionellosis, followed by L micdadei (otherwise known as the Pittsburgh pneumonia agent), L bozemanii, L dumoffii, and L longbeachae. Fifteen serogroups of L pneumophila have been identified, with serogroups 1, 4, and 6 being the primary causes of human disease. Serogroup 1 is thought to be responsible for 80% of the reported cases of legionellosis caused by L pneumophila. [5]
Patient education
For patient education information, see Bronchoscopy.
Pathophysiology
Legionella species are obligate or facultative intracellular parasites. Water is the major environmental reservoir for Legionella; the bacteria can infect and replicate within protozoa such as Acanthamoeba and Hartmannella, which are free-living amoebae found in natural and manufactured water systems. (Legionellae can resist low levels of chlorine used in water distribution systems.) [4]
Within the amebic cells, Legionella species can avoid the endosomal-lysosomal pathway and can replicate within the phagosome. Surviving and growing in amebic cells allows Legionella to persist in nature.
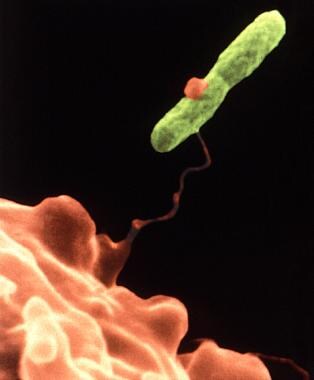 This electron micrograph depicts an amoeba, Hartmannella vermiformis (orange), as it entraps a Legionella pneumophila bacterium (green) with an extended pseudopod. After it is ingested, the bacterium can survive as a symbiont within what then becomes its protozoan host. The amoeba then becomes a so-called "Trojan horse," since, by harboring the pathogenic bacterium, the amoeba can afford it protection. In fact, in times of adverse environmental conditions, the amoeba can metamorphose into a cystic stage, enabling it, and its symbiotic resident, to withstand the environmental stress. Image courtesy of the Centers for Disease Control and Prevention and Dr. Barry S Fields.
This electron micrograph depicts an amoeba, Hartmannella vermiformis (orange), as it entraps a Legionella pneumophila bacterium (green) with an extended pseudopod. After it is ingested, the bacterium can survive as a symbiont within what then becomes its protozoan host. The amoeba then becomes a so-called "Trojan horse," since, by harboring the pathogenic bacterium, the amoeba can afford it protection. In fact, in times of adverse environmental conditions, the amoeba can metamorphose into a cystic stage, enabling it, and its symbiotic resident, to withstand the environmental stress. Image courtesy of the Centers for Disease Control and Prevention and Dr. Barry S Fields.
Legionella species infect human macrophages and monocytes; intracellular replication of the bacterium is observed within these cells in the alveoli. The intracellular infections of protozoa and macrophages have many similarities. [4]
Activated T cells produce lymphokines that stimulate increased antimicrobial activity of macrophages. This cell-mediated activation is key to halting the intracellular growth oflegionellae. The significant role of cellular immunity explains why legionellae are observed more frequently in immunocompromised patients. Humoral immunity is thought to play a secondary role in the host response to Legionella infection.
Etiology
Legionella transmission is thought to occur via inhalation of aerosolized mist from water sources, such as the following, that have been contaminated with the bacterium [4, 6, 7, 8, 9] :
-
Cooling systems
-
Showers
-
Decorative fountains
-
Humidifiers
-
Respiratory therapy equipment
-
Whirlpool spas
-
Ice machines
-
Potting soil (L longbeachae spp)
-
Compost (L longbeachae spp)
-
Roadside puddles
-
Tubs used for water births [10]
Legionnaires disease (LD) may be travel associated from exposure in aircraft or hotel facilities. Person-to-person transmission, however, has not been documented.
The highest incidence of LD occurs during late spring and early fall, when air-conditioning systems are used more frequently. [11, 12] Nosocomial acquisition likely occurs via aspiration, respiratory therapy equipment, [8] or contaminated water. In addition, transmission has been linked to the use of humidifiers, nebulizers, and items that were rinsed with contaminated tap water.
The following features increase the likelihood of colonization and amplification of legionellae in human-made water environments:
-
Temperature of 25-42°C
-
Stagnation
-
Scale and sediment
-
Presence of certain free-living aquatic amoebae capable of supporting intracellular growth of legionellae
Risk factors
The risk for infection increases with the type and intensity of the exposure, as well as the health status of the exposed individual. Numerous factors increase the risk of acquiring Legionella infections, including the following:
-
Advanced age
-
Smoking
-
Chronic heart or lung disease
-
Immunocompromised hosts with impaired cell-mediated immunity (eg, acquired immunodeficiency syndrome [AIDS]) or immunosuppressive medication use (especially corticosteroids)
-
Diabetes
-
Hematologic malignancies
-
End-stage renal disease
-
Alcohol abuse
Epidemiology
Occurrence in the United States
Legionnaires disease (LD) has a reported incidence of 8000-18,000 cases per year. In certain geographic areas, community-acquired LD is more common. Although LD is reportable in all 50 states, it is estimated that only 5-10% of cases are reported. Although most cases of the disease are sporadic, 10-20% are linked to outbreaks. Legionnaires disease is more common in the summer, especially in August, and is slightly more prevalent in the northern United Sates. [1, 2, 4]
Prevalence reports for Legionella have increased with time, likely due to the availability of more effective testing modalities. However, it also is possible that Legionella infections are increasing in frequency for environmental, population-based, or behavioral reasons.
Legionnaires disease is among the top 3-4 microbial causes of CAP, constituting approximately 1-9% of patients with CAP who require hospitalization. Legionnaires disease is an even more common cause of severe pneumonia in patients who require admission to an intensive care unit (ICU), ranking second, after pneumococcal pneumonia, in such cases. In addition, it is recognized as the most common cause of atypical pneumonia in hospitalized patients.
Legionnaires disease cases acquired in the hospital usually occur as outbreaks and most often result from the presence of Legionella in water sources and on surfaces (eg, pipes, rubber, plastics). The organism also is found in water sediment, which may explain its ability to persist despite flushing of hospital water systems. [13, 14]
Barsky et al compared epidemiological patterns of LD cases reported to the US Centers for Disease Control and Prevention before and during a rise in cases. The average age-standardized incidence was 0.48 cases per 100,000 population from 1992 to 2002, significantly increasing to 2.71 cases per 100,000 in 2018. Reported cases increased across all demographic groups, and the rise was more substantial in groups with initially higher incidence rates. White individuals accounted for the largest number of cases overall, but Black or African-American individuals had the highest incidence rate. Incidence and increases were most notable in the East North Central, Middle Atlantic, and New England regions. Seasonality, particularly in the Northeast and Midwest, became more pronounced from 2003 to 2018. The rising incidence of Legionnaires' disease was prominently linked to increasing racial disparities, geographical concentration, and seasonality. [15]
International occurrence
Legionnaires disease is thought to occur worldwide and to be the cause of 2-15% of all CAP cases that require hospitalization. Outbreaks have been recognized throughout North America, Africa, Australia, Europe, and South America.
Sex- and age-related demographics
Men have a greater risk of acquiring L pneumophila infection. Older age is another risk factor; the weighted mean age for patients with LD is 52.7 years, with increasing incidence until age 79 years. Mortality rates also are higher in older patients. The incidence of LD in persons younger than 35 years is less than 0.1 cases per 100,000 people.
Prognosis
Recovery is variable in Legionnaires disease (LD); some patients experience rapid improvement, whereas others have a much more protracted course despite treatment. The mortality rate approaches 50% with nosocomial infections. [4]
Progressive respiratory failure is the most common cause of death in patients with Legionella pneumonia. However, the mortality rate depends on the comorbid conditions of the patient, as well as on the choice and timeliness of antibiotics administration. The site of acquisition (eg, nosocomial, community-acquired) also may affect the outcome.
Complications
Potential complications include the following [4] :
-
Decreased pulmonary function
-
Fulminant respiratory failure
-
Dehydration, septic shock
-
Respiratory insufficiency, hypoxic respiratory failure
-
Endocarditis
-
Neurologic symptoms: Including lethargy, headache, altered mental status, and nonfocal neurologic examination findings
-
Gastrointestinal symptoms: Diarrhea, vomiting
-
Multiple organ failure
-
Coma
-
Bacteremia or abscess formation (in the lungs or at extrapulmonary sites) in immunocompromised patients
-
Death: In 10% of treated nonimmunocompromised patients and in as many as 80% of untreated immunocompromised patients
A study by van Loenhout et al that included 190 patients with LD found that a year after the disease’s onset, many patients were still suffering from 1 or more adverse health effects, particularly fatigue and reduced general quality of life. [16]
-
This electron micrograph depicts an amoeba, Hartmannella vermiformis (orange), as it entraps a Legionella pneumophila bacterium (green) with an extended pseudopod. After it is ingested, the bacterium can survive as a symbiont within what then becomes its protozoan host. The amoeba then becomes a so-called "Trojan horse," since, by harboring the pathogenic bacterium, the amoeba can afford it protection. In fact, in times of adverse environmental conditions, the amoeba can metamorphose into a cystic stage, enabling it, and its symbiotic resident, to withstand the environmental stress. Image courtesy of the Centers for Disease Control and Prevention and Dr. Barry S Fields.

