Background
Myocarditis is an inflammatory disease of the myocardium with a wide range of clinical presentations, from subtle to devastating. More specifically, it is described as "an inflammatory infiltrate of the myocardium with necrosis and/or degeneration of adjacent myocytes" [1] and it can be characterized on the basis of etiology, phase, severity, predominant symptoms, and pathological features. [2]
The image below depicts numerous lymphocytes with associated myocyte damage in acute myocarditis.
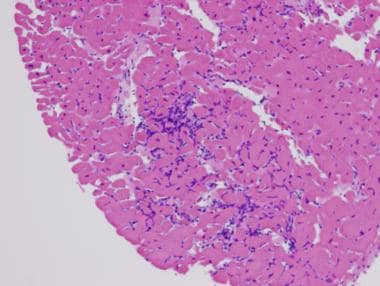 Myocarditis. Hematoxylin and eosin staining. Low power. This image shows numerous lymphocytes with associated myocyte damage. Photo courtesy of Dr Donald Weilbaecher.
Myocarditis. Hematoxylin and eosin staining. Low power. This image shows numerous lymphocytes with associated myocyte damage. Photo courtesy of Dr Donald Weilbaecher.
Myocarditis usually manifests in an otherwise healthy person and can result in rapidly progressive (and fatal) heart failure (HF) and arrhythmia. [3, 4] In the clinical setting, myocarditis is synonymous with inflammatory cardiomyopathy. It is diagnosed by established histologic, immunologic, and immunochemical criteria; however, electrocardiographic, biochemical, and advanced imaging studies can aid in diagnosis. (See Etiology, Presentation, and Workup.)
Definitions
The definition of myocarditis has evolved based on histologic, immunologic, and imaging criteria. The condition is broadly defined as inflammation of the myocardium, but different organizations and guidelines have proposed specific diagnostic criteria:
Histopathologic definition (Dallas Criteria, 1987) [1] : Myocarditis is defined as the presence of inflammatory infiltrates in the myocardium associated with myocyte necrosis that is not due to ischemic heart disease. However, this criteria rely on endomyocardial biopsy (EMB), which has low sensitivity due to sampling errors and does not capture cases without overt necrosis.
Clinicopathologic definition (Lieberman, 1991) [5] : Myocarditis is classified by its clinical presentation and clinical trajectories as follows:
-
Fulminant myocarditis: Follows a viral prodrome; distinct onset of illness consisting of severe cardiovascular compromise with ventricular dysfunction and multiple foci of active myocarditis; either resolves spontaneously or results in death. The American Heart Association (AHA) definition this condition as "a sudden and severe inflammation of the myocardium resulting in myocyte necrosis, edema, and cardiogenic shock." [6, 7]
-
Acute myocarditis: Less distinct onset of illness, with established ventricular dysfunction; may progress to dilated cardiomyopathy
-
Chronic active myocarditis: Less distinct onset of illness, with clinical and histologic relapses; development of ventricular dysfunction associated with chronic inflammatory changes (including giant cells)
-
Chronic persistent myocarditis: Less distinct onset of illness; persistent histologic infiltrate with foci of myocyte necrosis but without ventricular dysfunction (despite symptoms; eg, chest pain, palpitations)
Immunohistochemical definition (World Health Organization [WHO], Marburg, 1996 [8] ; updated in 2000) [9] : The Marburg criteria are based on the presence of more than 14 mononuclear leukocytes per mm2 on bioptic samples, [10] with the presence of over 7 T-lymphocytes per mm2. Subequent criteria expanded to include immunohistochemical staining for immune cells (eg, CD3+ T cells, CD68+ macrophages) and provided a more sensitive diagnosis than conventional histology alone.
European Society of Cardiology (ESC) definition (Caforio et al, 2013) [11] :Myocarditis is defined as an inflammatory disease of the myocardium diagnosed by EMB according to histologic, immunohistochemical, or molecular criteria. Probable myocarditis or clinically suspected myocarditis is defined by clinical presentation with supportive imaging (eg, cardiac magnetic resonance imaging [CMRI]) and laboratory findings (elevated troponin, electrocardiographic [ECG] changes). Infectious myocarditis versus autoimmune myocarditis was also introducted on the basis of etiology.
CMRI-based definition (Lake Louise Criteria, 2009 [12] ; updated in 2018 [13] ): Myocarditis is diagnosed by CMRI when there is myocardial inflammation demonstrated by T2-based markers for myocardial edema (T2-weighted imaging or T2 mapping) and T1-based markers of myocardial injury/necrosis (late gadolinium enhancement [LGE], T1 mapping, or extracellular volume [ECV]). The 2018 update refined these criteria by adding parametric mapping techniques, improving sensitivity.
Expert consensus definition (Ammirati, 2020) [2] : For those presenting with acute myocarditis, there are more specific features to classify myocarditis as complicated or uncomplicated:
-
Complicated myocarditis: Presenting with one or more of the following: left ventricular (LV) dysfunction (LV ejection fraction [EF] < 50% on first echocardiogram), sustained ventricular arrhythmias, advanced heart block, HF, low cardiac output syndrome, cardiogenic shock
-
Uncomplicated myocarditis: Presenting without the above manifestations of complicated myocarditis
Note: For virus-induced myocarditis, there is distinction between virus-mediated myocarditis(related to viral infection via direct viral cytotoxicity at the myocardial level) or virus-triggered myocarditis (immune-mediated lymphocytic myocarditis triggered by viral infection, often in the absence of viral genome). While direct viral injury initiates myocardial damage, the immune system's response can perpetuate inflammation, leading to chronic cardiomyopathy. [14]
These terms are still used to describe the clinical presentation and progression of myocarditis, particularly in the absence of ongoing histologic evaluation. (See Etiology and Presentation.)
Patient education
Patients should be advised of the current understanding of the natural history of myocarditis and the strengths and limitations of different diagnostic testing and therapeutic options. (See Etiology, Presentation, Workup, Treatment, and Medication.)
The Myocarditis Foundation is a nonprofit organization dedicated to helping those affected by myocarditis, as well as educating the public and medical communities on the disease and funding myocarditis-specific research.
The American Heart Association provides education materials and support for patients with myocarditis.
Etiology
Myocarditis is probably caused by a wide variety of infectious organisms, including in pediatric patients, autoimmune disorders, and exogenous agents, with genetic and environmental predisposition. [2] There remains debate about the specific mechanisms that govern "the transition from the initial trigger to myocardial inflammation and from acute myocardial damage to chronic ventricular dysfunction." [2] Most cases are presumed to be caused by a common pathway of host-mediated, autoimmune-mediated injury, although direct cytotoxic effects of the causative agent and damages due to cytokine expression in the myocardium may play some role in myocarditis etiology. Damage occurs through the following mechanisms:
-
Direct cytotoxic effect of the causative agent
-
Secondary immune response, which can be triggered by the causative agent
-
Cytokine expression in the myocardium (eg, tumor necrosis factor [TNF] ̶ alpha, nitric oxide synthase)
-
Aberrant induction of apoptosis [15]
In myocarditis related to coronavirus disease 2019 (COVID-19), cardiac inflammation is generally due to direct cardiac invasion with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or a result of the intense cytokine storm that often arises during the course of the disease. [16]
Myocardial damage has two main phases:
-
Acute phase (first 2 weeks): Myocyte destruction is a direct consequence of the offending agent, which causes cell-mediated cytotoxicity and cytokine release, contributing to myocardial damage and dysfunction; detection of the causal agent is uncommon during this stage.
-
Chronic phase (>2 weeks): Continuing myocyte destruction is autoimmune in nature, with associated abnormal expression of human leukocyte antigen (HLA) in myocytes (and, in the case of viral myocarditis, persistence of the viral genome in myocardium).
Viral myocarditis
In viral myocarditis, viral isolates differ in tissue tropism and virulence. For example, coxsackievirus A9 is a self-limiting myocarditis, whereas coxsackievirus B3 causes severe myocarditis resulting in a high mortality. The induction of the coxsackie-adenovirus receptor (CAR) and the complement deflecting protein decay accelerating factor (DAF, CD55) may allow efficient internationalization of the viral genome.
Viral replication may lead to further disruption of metabolism and to perturbation of inflammation and its response. Vasospasm induced by endothelial cell viral infection may also contribute to further damage. [17]
New evidence of dystrophin disruption by expression of enteroviral protease 2A points to yet another unique pathogenic mechanism. [18] In contrast, some viruses (such as parvovirus B19) may focus on pericapillary depositions, contributing to diastolic dysfunction rather than to direct myocyte destruction. Regardless, viral persistence provides the necessary stimuli for autoimmune or other inflammatory responses.
Idiopathic myocarditis
Approximately 50% of the time, myocarditis is classified as idiopathic, although a report by Klugman et al found that 82% of the pediatric cases studied were considered idiopathic. [19] The investigators also determined that 3% of cases in the study had a known bacterial or viral etiology, and that 6% of cases were related to other diseases.
In idiopathic cases, a viral etiology is often suspected but unproved, even with sophisticated immunohistochemical and genomic studies. Studies on patients with idiopathic dilated cardiomyopathy found evidence of viral particles in EMB specimens in up to two thirds of the patients. [20]
Causes
Causes of myocarditis include [21] :
-
Viral: Enterovirus, [15] coxsackie B, adenovirus, influenza, cytomegalovirus (CMV), poliomyelitis, Epstein-Barr virus (EBV), human immunodeficiency virus type 1 (HIV-1), viral hepatitis, mumps, rubeola, varicella, variola/vaccinia, arbovirus, respiratory syncytial virus (RSV), herpes simplex virus (HSV), yellow fever virus, rabies, parvovirus, severee acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
-
Rickettsial: Scrub typhus, Rocky Mountain spotted fever, Q fever
-
Bacterial: Diphtheria, tuberculosis, streptococci, meningococci, brucellosis, clostridia, staphylococci, streptococci, pneumococci, melioidosis, Mycoplasma pneumoniae, Legionella pneumophila, psittacosis, syphilis, tetanus, Klebsiella species (spp), Leptospira spp, Salmonella spp, Brucella spp
-
Spirochetal: Syphilis, leptospirosis/Weil disease, relapsing fever/Borrelia, Lyme disease
-
Fungal: Candidiasis, aspergillosis, cryptococcosis, histoplasmosis, actinomycosis, blastomycosis, coccidioidomycosis, mucormycosis
-
Protozoal: Chagas disease, toxoplasmosis, trypanosomiasis, malaria, leishmaniasis, balantidiasis, sarcosporidiosis, toxoplasmosis, amebiasis
-
Helminthic: Trichinosis, echinococcosis, schistosomiasis, heterophyiasis, cysticercosis, visceral larva migrans, filariasis, Toxocara canis
-
Bites/stings: Scorpion venom, snake venom, black widow spider venom, wasp venom, tick paralysis
-
Drugs (usually causing hypersensitivity myocarditis)
Chemotherapeutic drugs: Doxorubicin and anthracyclines, streptomycin, cyclophosphamide, interleukin-2, anti-HER-2 receptor antibody/Herceptin, immune checkpoint inhibitors
Antibiotics: Penicillin, chloramphenicol, sulfonamides, tetracycline
Antihypertensive drugs: Methyldopa, spironolactone
Antiseizure drugs: Phenytoin, carbamazepine
Amphetamines, cocaine, catecholamines
Other drugs: Phenylbutazone, indomethacin (NSAIDs); acetazolamide (diuretic); amphotericin B (antifungal); isoniazid (antituberculin)
-
Chemicals: Hydrocarbons, carbon monoxide, arsenic, lead, phosphorus, mercury, cobalt
-
Physical agents (radiation, heatstroke, hypothermia)
-
Acute rheumatic fever
-
Systemic inflammatory disease: Giant cell myocarditis, sarcoidosis, Kawasaki disease, Crohn disease, systemic lupus erythematosus, ulcerative colitis, Wegener granulomatosis, thyrotoxicosis, scleroderma, rheumatoid arthritis, dermatomyositis
-
Peripartum cardiomyopathy
-
Posttransplant cellular rejection
-
Idiopathic
Some common causes of uncommon nonviral myocarditis
Chagas disease
Chagas disease is caused by a protozoal infestation with T cruzi. It is very uncommon in North America but can affect as much as half the population in endemic areas, such as South America. [22] Acute infection can cause protracted heart failure and death. Endomyocardial biopsy and microscopic examination typically demonstrate organisms, neutrophils, lymphocytes, macrophages, and eosinophils.
Giant cell myocarditis
In giant cell myocarditis, giant cells are present in the myocardium with or without granulomas. This type of myocarditis may be evidenced with tuberculosis, syphilis, rheumatoid arthritis, or rheumatic heart disease, or with fungal or parasitic infections. The characteristic cell is probably histiocytic in origin and is usually found in nonviral myocarditis. Similar cells have been noted in patients with myocarditis associated with drugs such as phenylbutazone. This type of myocarditis may not be specific [23] but, rather, may represent a final common pathologic pathway.
Systemic lupus erythematosus
Patients with SLE may have myocardial fibrinoid lesions in the connective tissue, with an accompanying cellular reaction. This reaction may also affect the cardiac valves, most notably the mitral and aortic valves. [24] Although the predominant cardiac manifestation of SLE is pericarditis, myocardial involvement with congestive heart failure (CHF) can occur. The treatment of choice is corticosteroids.
Kawasaki disease
Kawasaki disease may include myocardial involvement, which usually occurs in the acute phase; it can occur independent of any coronary artery involvement and usually resolves completely. In some cases, however, the diffuse myocarditis may be severe, and it may lead to heart failure and death. Although the pathophysiologic mechanisms that occur in Kawasaki disease are not known, a generalized autoimmune disorder is suspected.
Dermatomyositis
Dermatomyositis, a multisystem (probably autoimmune) disease, is characterized by diffuse, nonsuppurative inflammation of skeletal muscle and skin. However, cardiac involvement, including a loss of striations, fragmentation, and vascularization of muscle fibers, has been reported. The interstitium may also show a variable amount of edema. Tachycardia and conduction abnormalities are described, but heart failure is uncommon. Long-term therapy with prednisone is beneficial in most cases. Children have a good long-term prognosis, as most recover, and medication may be discontinued in 1-2 years.
Pathophysiology
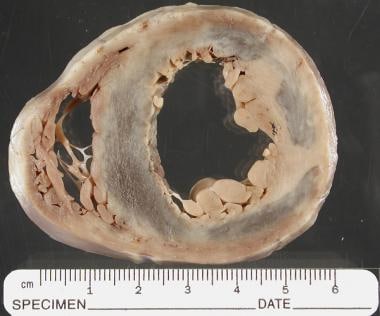
The pathophysiology of myocarditis involves a complex interplay of immunologic, genetic, and environmental factors that drive myocardial inflammation and the subsequent progression to chronic cardiomyopathy. Systolic function of the LV with concomitant dilatation of the heart and cardiac enlargement occurs, with an overall result of an attendant reduction in cardiac output. Moreover, progressive congestive HF (CHF) with elevated LV end-diastolic volume and pressure leads to a rise in left atrial pressure, which is transmitted into the pulmonary venous system; this may also result in pulmonary edema secondary to increased pulmonary arterial hydrostatic forces. Finally, during the healing stages of myocarditis, fibroblasts may replace existing myocytes with resultant scar formation. Chronic CHF may develop. An autopsy sample that shows scarring in the ventricles is seen below.
Infectious triggers and viral mechanisms
Viral infections are among the most common triggers of myocarditis, with viruses like coxsackievirus, adenovirus, parvovirus B19, and others directly infecting cardiac myocytes. The initial viral invasion of heart cells typically elicits an innate immune response, including activation of toll-like receptors (TLRs) on cardiac cells. This response leads to the release of proinflammatory cytokines (eg, TNF-α, IL-1, IL-6) and chemokines, which recruit immune cells to the site of infection. If the virus is not efficiently cleared, the ongoing immune response can progress from an acute to a chronic inflammatory phase, often marked by T-cell activation, antibody production, and, in some cases, an autoimmunity-driven attack against cardiac cells.
Genetic susceptibility and myocardial Injury
Genetic predispositions play a significant role in how an individual responds to myocarditis triggers. Variants in certain genes associated with dilated cardiomyopathy (eg, desmosomal, sarcomeric, and cytoskeletal genes) are known to increase the likelihood of progression to chronic cardiomyopathy following myocarditis. For example, pathogenic variants in desmosomal genes (eg, PKP2, DSP, FLNC) are implicated in arrhythmogenic cardiomyopathy, where inflammation and fibrosis result in both structural and electrical instability in the heart. These genetic susceptibilities may also influence the immune response by altering the expression of immune-regulatory genes in cardiac cells, predisposing the heart to autoimmune reactions.
Immune system dysregulation and autoimmunity
Both the innate and adaptive immune systems are dysregulated. The transition from an acute inflammatory response to chronic autoimmune myocarditis often involves molecular mimicry, in which viral antigens resemble self-antigens in the heart (such as myosin), leading to an autoimmune attack against cardiac tissues. During this process, CD4+ T helper (Th) cells, especially Th1 and Th17 subtypes, become polarized and contribute to sustained inflammation. The Th17 pathway, characterized by the production of IL-17 and other cytokines, is crucial in maintaining this chronic inflammation. Th17 cells are also known for their ability to recruit neutrophils and monocytes, perpetuating the cycle of inflammation and tissue damage.
Role of regulatory T cells and immune checkpoints
Regulatory T cells (Tregs) and immune checkpoints play essential roles in maintaining immune tolerance to prevent autoimmune responses against cardiac tissue. Tregs typically secrete anti-inflammatory cytokines such as IL-10 and TGF-β, which inhibit the activation of self-reactive T-cells. In myocarditis, however, the regulatory pathways are often overwhelmed or dysfunctional, resulting in an insufficient Treg response and failure to suppress autoreactive T cells. Immune checkpoints such as CTLA-4 and PD-1/PD-L1 are also disrupted in some myocarditis cases. Checkpoint inhibitor therapies used in cancer treatments, for example, can lead to myocarditis by blocking these pathways, effectively unleashing autoreactive T cells on the heart.
Cytokine storm and myocardial injury
In some cases of severe myocarditis, a “cytokine storm” may develop, with high levels of proinflammatory cytokines (eg, IL-1, IL-6, TNF-α, and IFN-γ) flooding the cardiac tissue. This cytokine storm can drive significant myocardial injury by exacerbating oxidative stress and endothelial dysfunction. Additionally, the influx of immune cells (especially macrophages and neutrophils) can release reactive oxygen species (ROS) and proteolytic enzymes, causing further cellular injury and necrosis. These damaging processes contribute to structural remodeling of the heart, fibrosis, and a decline in cardiac function.
Myocardial fibrosis and cardiac remodeling
The persistence of inflammation leads to fibroblast activation, which results in the deposition of collagen and formation of scar tissue within the myocardium. This fibrosis disrupts normal electrical conduction, increases the risk of arrhythmias, and contributes to a stiffer, less compliant myocardium. Over time, the heart undergoes structural remodeling, with LV dilatation and wall thinning. This remodeling compromises systolic and diastolic function, creating a chronic, progressive form of cardiomyopathy.
Extracellular vesicles and microRNA dysregulation
Relatively recent research has identified extracellular vesicles (EVs) and microRNAs (miRNAs) as important mediators in myocarditis. EVs released from injured cardiac cells can contain inflammatory miRNAs, which contribute to immune cell recruitment and further propagation of inflammation. For instance, miRNA-155 is upregulated in myocarditis and plays a role in Th17 cell polarization. Other miRNAs, such as mmu-miR-721 (in animal models), are investigated as potential biomarkers for diagnosing myocarditis and understanding its pathophysiology.
Gut-heart axis and microbiota-driven inflammation
Emerging evidence suggests a role of the gut microbiota in myocarditis. Certain gut-derived peptides from commensal bacteria such as Bacteroides can mimic cardiac myosin and activate autoreactive T-cells. This “gut-heart axis” is an area of interest, as dysbiosis or altered gut microbiota composition may exacerbate myocarditis by enhancing Th17 responses or reducing regulatory T-cell function. Factors such as diet, antibiotic use, and genetic predisposition can alter gut microbiota and potentially influence myocarditis progression.
Endothelial dysfunction and microvascular injury
Myocarditis often involves endothelial cell activation and microvascular injury, contributing to ischemic injury and fibrosis. Activated endothelial cells express adhesion molecules (eg, ICAM-1, VCAM-1) that recruit immune cells to the myocardium. Additionally, endothelial cells can release ROS and proinflammatory cytokines, exacerbating local inflammation. Persistent endothelial dysfunction can lead to capillary rarefaction, reducing oxygen supply to cardiac tissue and promoting fibrosis.
Epidemiology
United States data
The frequency of myocarditis is difficult to ascertain, owing to the wide variation of clinical presentation. Incidence is usually estimated at 1-10 cases per 100,000 persons, and it is higher in young men (and to some degree middle-aged women). Incidence of positive right ventricular biopsy findings in patients with suspected myocarditis is highly variable (range: 0-80%). According to estimates, as many as 1-5% of patients with acute viral infections may have involvement of the myocardium.
The availability of CMRI has expanded the ability to detect myocarditis in patients who might otherwise not receive an EMB. Consequently, the reported incidence of myocarditis has risen from roughly 1-10 cases per 100,000 persons to around 9.5-14.4 cases per 100,000, paralleling CMRI's more widespread use.
International data
A population study of more than 670,000 healthy young male military recruits in Finland found that 98 cases had myocarditis mimicking myocardial ischemia, 1 case presented as sudden death, and 9 cases presented as recent-onset dilated cardiomyopathy. [25, 26]
A Japanese 20-year series of 377,841 autopsies found idiopathic, nonspecific, interstitial, or viral myocarditis in only 0.11% of individuals. [27]
Race-, sex-, and age-related demographics
No particular race predilection is noted for myocarditis except for peripartum cardiomyopathy (a specific form of myocarditis that appears to have a higher incidence in patients of African descent) and cardiac sarcoidosis (which affects US Black populations more than White populations). [28] The incidence of myocarditis is similar between males and females, although young males are particularly susceptible.
Patients are usually fairly young, most often affecting younger adults aged 20-40 years. [28] However, when it occurs in children, this patient population appears to have a more severe presentation than that of adults, with a higher percentage requiring temporary mechanical circulatory support. [28] In addition, the elderly may be underdiagnosed. The median age of patients affected with lymphocytic myocarditis is 42 years. Patients with giant cell myocarditis may be older (mean age: 58 years), but this condition usually does not discriminate with respect to age, sex, or presenting symptoms.
Other susceptible groups include immunocompromised individuals, pregnant women, and children (particularly neonates).
Prognosis
The prognosis for myocarditis varies and depends on age, etiology, and severity at the time of presentation.
Patients with fulminant myocarditis have a high mortality risk when the condition is not recognized and treated early; death occurs from cardiogenic shock, fatal ventricular tachyarrhythmias, or bradycardia. [6] Prompt recognition and initiation of circulatory support and maintenance of end-organ function are key to a favorable outcome. [6]
Those who survive fulminant myocarditis have a good prognosis. In a study of 147 cases of myocarditis monitored for an average of 5.6 years, 93% of the 15 patients with fulminant disease were alive without transplant 11 years after biopsy, compared with 45% of the 132 patients with less severe disease. [29, 30] LV dilatation was not as severe in the fulminant cases as in the nonfulminant ones.
Expression of soluble Fas and Fas ligands at initial presentation appears to be a good serologic marker to predict the prognosis of acute myocarditis, whereas anti-myosin autoantibodies are associated with development of worse cardiac dysfunction in chronic myocarditis. [31]
Predictors of death or need for heart transplantation after acute myocarditis in multivariate analyses include syncope, low EF, and left bundle-branch block—all indicators of advanced cardiomyopathy. [32]
Morbidity and mortality
Most patients with mild symptoms recover completely without any residual cardiac dysfunction, although one third subsequently develop dilated cardiomyopathy. [26, 33, 34, 35] Eosinophilic myocarditis, left undiagnosed, can result in progressive, irreversible, and fatal myocardial damage. [36] Cardiogenic shock may occur in fulminant cases of myocarditis.
The mortality in newborn infants is high (75%) in some reports. Older infants and children have a better prognosis, with mortality ranging from 10% to 25% in clinically recognizable cases. Some studies have suggested complete recovery in as many as 50% of the cases.
In the Myocarditis Treatment Trial, the 1-year mortality was 20% and the 4-year mortality was 56% in a population with symptomatic HF presentation and LVEF lower than 45% at baseline. [37] Severe heart block requiring permanent pacemaker placement occurred in 1% of patients in the trial.
In a study of patients with giant cell myocarditis, 89% of patients either died or underwent cardiac transplantation, with a median survival from symptom onset to death or transplantation of only 5.5 months. [38]
Klugman et al reported a 92% survival among 216 pediatric patients with myocarditis. [19] According to the investigators, nonsurviving patients were characterized by a greater severity of illness at presentation and a frequent need for extracorporeal membrane oxygenation and other intensive care unit therapies. With regard to postpartum cardiomyopathy, the mortality at 1 year can be as high as 50%.
-
Myocarditis. Hematoxylin and eosin staining. Low power. This image shows numerous lymphocytes with associated myocyte damage. Photo courtesy of Dr Donald Weilbaecher.
-
Myocarditis. Hematoxylin and eosin staining. High power. Toxoplasmosis (numerous purple granular-like structures within a myocyte) is demonstrated.
-
Myocarditis. Hematoxylin and eosin staining. High power. Lymphocytes, histiocytes, and a multinucleated giant cell representing sarcoidosis (a diagnosis of exclusion) is shown.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Medication
- Medication Summary
- Angiotensin Receptor-Neprilysin Inhibitors (ARNi)
- ACE inhibitors
- Diuretics
- Angiotensin II receptor blockers
- Antidiabetics, SGLT2 Inhibitors
- Beta-adrenergic blockers
- Aldosterone Antagonists, Selective
- Vasodilators
- Inotropic agents
- Anti-Inflammatory Agents
- Immunosuppressants
- Show All
- Media Gallery
- References