Practice Essentials
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, causing 3.23 million deaths in 2019. Tobacco smoking accounts for over 70% of COPD cases in high-income countries. In low- and middle-income countries, tobacco smoking accounts for only 30–40% of COPD cases, and household air pollution is a major risk factor. [1]
Patients typically have symptoms of chronic bronchitis and emphysema, but the classic triad also includes asthma or a combination of the above (see the image below).
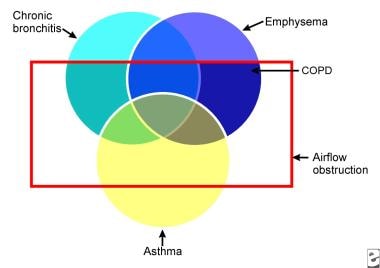 Venn diagram of chronic obstructive pulmonary disease (COPD). Chronic obstructive lung disease is a disorder in which subsets of patients may have dominant features of chronic bronchitis, emphysema, or asthma. The result is airflow obstruction that is not fully reversible.
Venn diagram of chronic obstructive pulmonary disease (COPD). Chronic obstructive lung disease is a disorder in which subsets of patients may have dominant features of chronic bronchitis, emphysema, or asthma. The result is airflow obstruction that is not fully reversible.
Chronic bronchitis is defined clinically as the presence of a chronic productive cough for 3 months during each of 2 consecutive years (other causes of cough being excluded).
Emphysema is defined pathologically as an abnormal, permanent enlargement of the air spaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis.
The 2022 International Classification of Diseases (ICD)-10 diagnosis code for COPD, unspecified is J44.9.
Signs and symptoms
Patients typically present with a combination of signs and symptoms of chronic bronchitis, emphysema, and reactive airway disease. Symptoms include the following:
-
Cough, usually worse in the mornings and productive of a small amount of colorless sputum
-
Breathlessness: The most significant symptom, but usually does not occur until the sixth decade of life
-
Wheezing: May occur in some patients, particularly during exertion and exacerbations
While the sensitivity of physical examination in detecting mild-to-moderate COPD is relatively poor, the physical signs are quite specific and sensitive for severe disease. Findings in severe disease include the following:
-
Tachypnea and respiratory distress with simple activities
-
Use of accessory respiratory muscles and paradoxical indrawing of lower intercostal spaces (Hoover sign)
-
Cyanosis
-
Elevated jugular venous pulse (JVP)
-
Peripheral edema
Thoracic examination reveals the following:
-
Hyperinflation (barrel chest)
-
Wheezing – Frequently heard on forced and unforced expiration
-
Diffusely decreased breath sounds
-
Hyperresonance on percussion
-
Prolonged expiration
-
Coarse crackles beginning with inspiration in some cases
Certain characteristics allow differentiation between disease that is predominantly chronic bronchitis and that which is predominantly emphysema. Chronic bronchitis characteristics include the following:
-
Patients may be obese
-
Frequent cough and expectoration are typical
-
Use of accessory muscles of respiration is common
-
Coarse rhonchi and wheezing may be heard on auscultation
-
Patients may have signs of right heart failure (ie, cor pulmonale), such as edema and cyanosis
Emphysema characteristics include the following:
-
Patients may be very thin with a barrel chest
-
Patients typically have little or no cough or expectoration
-
Breathing may be assisted by pursed lips and use of accessory respiratory muscles; patients may adopt the tripod sitting position
-
The chest may be hyperresonant, and wheezing may be heard
-
Heart sounds are very distant
See Clinical Presentation for more detail.
Diagnosis
The formal diagnosis of COPD is made with spirometry; when the ratio of forced expiratory volume in 1 second over forced vital capacity (FEV1/FVC) is less than 70% of that predicted for a matched control, it is diagnostic for a significant obstructive defect. Criteria for assessing the severity of airflow obstruction (based on the percent predicted postbronchodilator FEV1) are as follows:
-
Stage I (mild): FEV1 80% or greater of predicted
-
Stage II (moderate): FEV1 50-79% of predicted
-
Stage III (severe): FEV1 30-49% of predicted
-
Stage IV (very severe): FEV1 less than 30% of predicted or FEV1 less than 50% and chronic respiratory failure
Arterial blood gas (ABG) findings are as follows:
-
ABGs provide the best clues as to acuteness and severity of disease exacerbation
-
Patients with mild COPD have mild to moderate hypoxemia without hypercapnia
-
As the disease progresses, hypoxemia worsens and hypercapnia may develop, with the latter commonly being observed as the FEV1 falls below 1 L/s or 30% of the predicted value
-
pH usually is near normal; a pH below 7.3 generally indicates acute respiratory compromise
-
Chronic respiratory acidosis leads to compensatory metabolic alkalosis
In patients with emphysema, frontal and lateral chest radiographs reveal the following:
-
Flattening of the diaphragm
-
Increased retrosternal air space
-
A long, narrow heart shadow
-
Rapidly tapering vascular shadows accompanied by hyperlucency of the lungs
-
Radiographs in patients with chronic bronchitis show increased bronchovascular markings and cardiomegaly
Advantages of high-resolution CT include the following:
-
Greater sensitivity than standard chest radiography
-
High specificity for diagnosing emphysema (outlined bullae are not always visible on a radiograph)
-
May provide an adjunctive means of diagnosing various forms of COPD (eg, lower lobe disease may suggest alpha1-antitrypsin (AAT) deficiency
-
May help the clinician determine whether surgical intervention would benefit the patient
Other tests are as follows:
-
Hematocrit – Patients with polycythemia (hematocrit greater than 52% in men or 47% in women) should be evaluated for hypoxemia at rest, with exertion, or during sleep
-
Serum potassium – Diuretics, beta-adrenergic agonists, and theophylline act to lower potassium levels
-
Measure AAT in all patients younger than 40 years, in those with a family history of emphysema at an early age, or with emphysematous changes in a nonsmoker (also see Alpha1-Antitrypsin Deficiency).
-
Sputum evaluation will show a transformation from mucoid in stable chronic bronchitis to purulent in acute exacerbations
-
Pulse oximetry, combined with clinical observation, provides instant feedback on a patient's status
-
Electrocardiography can help establish that hypoxia is not resulting in cardiac ischemia and that the underlying cause of respiratory difficulty is not cardiac in nature
-
Two-dimensional echocardiography can screen for pulmonary hypertension
-
Right-sided heart catheterization can confirm pulmonary artery hypertension and gauge the response to vasodilators
See Workup for more detail.
Management
Smoking cessation continues to be the most important therapeutic intervention for COPD. Risk factor reduction (eg, influenza vaccine) is appropriate for all stages of COPD. Approaches to management by stage include the following:
-
Stage I (mild obstruction): Short-acting bronchodilator as needed
-
Stage II (moderate obstruction): Short-acting bronchodilator as needed; long-acting bronchodilator(s); cardiopulmonary rehabilitation
-
Stage III (severe obstruction): Short-acting bronchodilator as needed; long-acting bronchodilator(s); cardiopulmonary rehabilitation; inhaled glucocorticoids if repeated exacerbations
-
Stage IV (very severe obstruction or moderate obstruction with evidence of chronic respiratory failure): Short-acting bronchodilator as needed; long-acting bronchodilator(s); cardiopulmonary rehabilitation; inhaled glucocorticoids if repeated exacerbation; long-term oxygen therapy (if criteria met); consider surgical options such as lung volume reduction surgery (LVRS) and lung transplantation
Agents used include the following:
-
Short-acting beta2 -agonist bronchodilators (eg, albuterol, metaproterenol, levalbuterol, pirbuterol)
-
Long-acting beta2 -agonist bronchodilators (eg, salmeterol, formoterol, arformoterol, indacaterol, vilanterol)
-
Respiratory anticholinergics (eg, ipratropium, tiotropium, aclidinium, revefenacin)
-
Xanthine derivatives (ie, theophylline)
-
Phosphodiesterase-4 Inhibitors (ie, roflumilast)
-
Inhaled corticosteroids (eg, fluticasone, budesonide): Peripheral blood eosinophil counts may help stratify the likelihood of efficacy.
-
Oral corticosteroids (eg, prednisone)
-
Beta2 -agonist and anticholinergic combinations (eg, ipratropium and albuterol, umeclidinium bromide/vilanterol inhaled)
-
Beta2 -agonist and corticosteroid combinations (eg, budesonide/formoterol, fluticasone and salmeterol, vilanterol/fluticasone inhaled)
Pulmonary rehabilitation programs are typically multidisciplinary approaches that emphasize the following:
-
Patient and family education
-
Smoking cessation
-
Medical management (including oxygen and immunization)
-
Respiratory and chest physiotherapy
-
Physical therapy with bronchopulmonary hygiene, exercise, and vocational rehabilitation
-
Psychosocial support
Indications for admission for acute exacerbations include the following:
-
Failure of outpatient treatment
-
Marked increase in dyspnea
-
Altered mental status
-
Increase in hypoxemia or hypercapnia
-
Inability to tolerate oral medications such as antibiotics or steroids
See Treatment and Medication for more detail.
Background
In Western Europe, Badham (1808) and Laennec (1827) made the classic descriptions of chronic bronchitis and emphysema in the early 19th century. A British medical textbook of the 1860s described the familiar clinical picture of chronic bronchitis as an advanced disease with repeated bronchial infections that ended in right-sided heart failure. Overall, this malady caused more than 5% of all deaths in the Middle Ages and earlier. The condition was most common among the poor; therefore, it was attributed to "bad" living.
Developments in the 20th century included the widespread use of spirometry (see Workup), recognition of airflow obstruction as a key factor in determining disability, and the improvement of pathologic methods to assess emphysema. Participants in the Ciba symposium of 1958 proposed definitions of chronic bronchitis and emphysema, incorporating the concept of airflow obstruction.
Chronic bronchitis is defined clinically as the presence of a chronic productive cough for 3 months during each of 2 consecutive years (other causes of cough being excluded). Emphysema, on the other hand, is defined pathologically as an abnormal, permanent enlargement of the air spaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis.
Airflow limitation in emphysema is due to loss of elastic recoil and decrease in airway tethering, whereas chronic bronchitis leads to narrowing of airway caliber and increase in airway resistance. Although some patients predominantly display signs of one of these diseases or the other, most fall somewhere in between the spectrum of these two conditions.
Oral and inhaled medications are used for patients with stable COPD to reduce dyspnea, improve exercise tolerance, and prevent complications. Most of the medications used in COPD treatment are directed at the potentially reversible mechanisms of airflow limitation. (See Medication.)
Pathophysiology
Pathologic changes in chronic obstructive pulmonary disease (COPD) occur in the large (central) airways, the small (peripheral) bronchioles, and the lung parenchyma. Most cases of COPD are the result of exposure to noxious stimuli, most often cigarette smoke. The normal inflammatory response is amplified in persons prone to COPD development. The pathogenic mechanisms are not clear but are most likely diverse. Increased numbers of activated polymorphonuclear leukocytes and macrophages release elastases in a manner that cannot be counteracted effectively by antiproteases, resulting in lung destruction.
The primary offender has been found to be human leukocyte elastase, with synergistic roles suggested for proteinase-3 and macrophage-derived matrix metalloproteinases (MMPs), cysteine proteinases, and a plasminogen activator. Additionally, increased oxidative stress caused by free radicals in cigarette smoke, the oxidants released by phagocytes, and polymorphonuclear leukocytes all may lead to apoptosis or necrosis of exposed cells. Accelerated aging and autoimmune mechanisms have also been proposed as having roles in the pathogenesis of COPD. [4, 5]
Cigarette smoke causes neutrophil influx, which is required for the secretion of MMPs; this suggests, therefore, that neutrophils and macrophages are required for the development of emphysema.
Studies have also shown that in addition to macrophages, T lymphocytes, particularly CD8+, play an important role in the pathogenesis of smoking-induced airflow limitation.
To support the inflammation hypothesis further, a stepwise increase in alveolar inflammation has been found in surgical specimens from patients without COPD versus patients with mild or severe emphysema. Indeed, mounting evidence supports the concept that dysregulation of apoptosis and defective clearance of apoptotic cells by macrophages play a prominent role in airway inflammation, particularly in emphysema. [6] Azithromycin (Zithromax) has been shown to improve this macrophage clearance function, providing a possible future treatment modality. [7]
In patients with stable COPD without known cardiovascular disease, there is a high prevalence of microalbuminuria, which is associated with hypoxemia independent of other risk factors. [8]
Chronic bronchitis
Mucous gland hyperplasia (as seen in the images below) is the histologic hallmark of chronic bronchitis. Airway structural changes include atrophy, focal squamous metaplasia, ciliary abnormalities, variable amounts of airway smooth muscle hyperplasia, inflammation, and bronchial wall thickening.
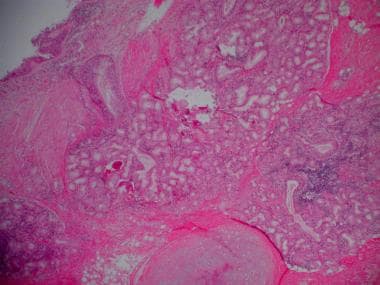 Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells.
Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells.
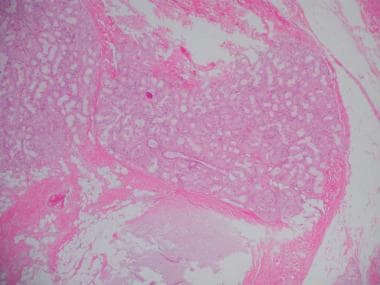 Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells (high-powered view).
Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells (high-powered view).
Damage to the endothelium impairs the mucociliary response that clears bacteria and mucus. Inflammation and secretions provide the obstructive component of chronic bronchitis. Neutrophilia develops in the airway lumen, and neutrophilic infiltrates accumulate in the submucosa. The respiratory bronchioles display a mononuclear inflammatory process, lumen occlusion by mucus plugging, goblet cell metaplasia, smooth muscle hyperplasia, and distortion due to fibrosis. These changes, combined with loss of supporting alveolar attachments, cause airflow limitation by allowing airway walls to deform and narrow the airway lumen.
In contrast to emphysema, chronic bronchitis is associated with a relatively undamaged pulmonary capillary bed. The body responds by decreasing ventilation and increasing cardiac output. This V/Q mismatch results in rapid circulation in a poorly ventilated lung, leading to hypoxemia and polycythemia. Eventually, hypercapnia and respiratory acidosis develop, leading to pulmonary artery vasoconstriction and cor pulmonale. With the ensuing hypoxemia, polycythemia, and increased CO2 retention, these patients have signs of right heart failure and are known as "blue bloaters."
Emphysema
Emphysema is a pathologic diagnosis defined by permanent enlargement of airspaces distal to the terminal bronchioles. This leads to a dramatic decline in the alveolar surface area available for gas exchange. Furthermore, loss of alveoli leads to airflow limitation by 2 mechanisms. First, loss of the alveolar walls results in a decrease in elastic recoil, which leads to airflow limitation. Second, loss of the alveolar supporting structure leads to airway narrowing, which further limits airflow.
Emphysema has 3 morphologic patterns:
-
Centriacinar
-
Panacinar
-
Distal acinar, or paraseptal
Centriacinar emphysema is characterized by focal destruction limited to the respiratory bronchioles and the central portions of the acini. This form of emphysema is associated with cigarette smoking and is typically most severe in the upper lobes.
Panacinar emphysema involves the entire alveolus distal to the terminal bronchiole. The panacinar type is typically most severe in the lower lung zones and generally develops in patients with homozygous alpha1-antitrypsin (AAT) deficiency.
Distal acinar emphysema, or paraseptal emphysema, is the least common form and involves distal airway structures, alveolar ducts, and sacs. This form of emphysema is localized to fibrous septa or to the pleura and leads to formation of bullae (as seen in the images below). The apical bullae may cause pneumothorax. Paraseptal emphysema is not associated with airflow obstruction.
The gradual destruction of alveolar septae (shown in the image below) and of the pulmonary capillary bed in emphysema leads to a decreased ability to oxygenate blood. The body compensates with lowered cardiac output and hyperventilation. This V/Q mismatch results in relatively limited blood flow through a fairly well oxygenated lung with normal blood gases and pressures in the lung, in contrast to the situation in chronic bronchitis. Because of low cardiac output, the rest of the body also suffers from tissue hypoxia and pulmonary cachexia. Eventually, these patients develop muscle wasting and weight loss and are identified as "pink puffers."
Emphysematous destruction and small airway inflammation
Emphysematous destruction and small airway inflammation often are found in combination in individual patients, leading to the spectrum that is known as COPD. When emphysema is moderate or severe, loss of elastic recoil, rather than bronchiolar disease, is the dominant mechanism of airflow limitation. By contrast, when emphysema is mild, bronchiolar abnormalities are most responsible for the majority of the deficit in lung function. Although airflow obstruction in emphysema is often irreversible, bronchoconstriction due to inflammation accounts for some reversibility. Airflow limitation is not the only pathophysiologic mechanism by which symptoms occur.
Dynamic hyperinflation
Lung volumes, particularly dynamic hyperinflation, have also been shown to play a crucial role in the development of dyspnea perceived during exercise. In fact, the improvement in exercise capacity brought about by several treatment modalities, including bronchodilators, oxygen therapy, lung volume reduction surgery (LVRS), and maneuvers learned in pulmonary rehabilitation, is more likely due to delaying dynamic hyperinflation rather than improving the degree of airflow obstruction. [9, 10, 11, 12, 13, 14, 15, 16] Additionally, hyperinflation (defined as the ratio of inspiratory capacity to total lung capacity [IC/TLC]) has been shown to predict survival better than forced expiratory volume in 1 second (FEV1). [6]
Etiology
Cigarette smoking
The primary cause of COPD is exposure to tobacco smoke. Overall, tobacco smoking accounts for as much as 90% of COPD risk.
Cigarette smoking induces macrophages to release neutrophil chemotactic factors and elastases, which lead to tissue destruction. Clinically significant COPD develops in 15% of cigarette smokers, although this number is believed to be an underestimate. Age of initiation of smoking, total pack-years, and current smoking status predict COPD mortality.
People who smoke have an increased annual decline in FEV1: the physiologic normal decline in FEV1 is estimated to be 20-30 ml/y, but the rate of decline in COPD patients is generally 60 ml/y or greater.
Secondhand smoke, or environmental tobacco smoke, increases the risk of respiratory infections, augments asthma symptoms, and causes a measurable reduction in pulmonary function.
A study by Nagelmann et al concluded that lung function deviation and lung structural changes are present in people who smoke cigarettes before the clinical signs of airway obstruction reveal them. [17] These changes can be detected by body plethysmography and diffusing capacity measurement with routine spirometry.
Airway hyperresponsiveness
Airway hyperresponsiveness (ie, Dutch hypothesis) stipulates that patients who have nonspecific airway hyperreactivity and who smoke are at increased risk of developing COPD with an accelerated decline in lung function. Nonspecific airway hyperreactivity is inversely related to FEV1 and may predict a decline in lung function.
The data regarding the possible role of airway hyperresponsiveness as a risk factor for the development of COPD in people who smoke are unclear. It is important to note, however, that 60% demonstrate bronchial hyperresponsiveness. [18] Moreover, bronchial hyperreactivity may result from airway inflammation observed with the development of smoking-related chronic bronchitis. This may contribute to airway remodeling, leading to a more fixed obstruction, as is seen in persons with COPD.
Environmental factors
COPD does occur in individuals who have never smoked. [19] Although the role of air pollution in the etiology of COPD is unclear, the effect is small when compared with that of cigarette smoking. In developing countries, the use of biomass fuels with indoor cooking and heating is likely to be a major contributor to the worldwide prevalence of COPD. Long-term exposure to traffic-related air pollution may be a factor in COPD in patients with diabetes and asthma. [20]
Alpha1-antitrypsin deficiency
Alpha1-antitrypsin (AAT) is a glycoprotein member of the serine protease inhibitor family that is synthesized in the liver and is secreted into the bloodstream. The main purpose of this 394-amino-acid, single-chain protein is to neutralize neutrophil elastase in the lung interstitium and to protect the lung parenchyma from elastolytic breakdown. Severe AAT deficiency predisposes to unopposed elastolysis with the clinical sequela of an early onset of panacinar emphysema. To see complete information on Alpha1-Antitrypsin Deficiency, please go to the main article by clicking here.
AAT deficiency is the only known genetic risk factor for developing COPD and accounts for less than 1% of all cases in the United States. Severe AAT deficiency leads to premature emphysema at an average age of 53 years for nonsmokers and 40 years for smokers.
Nearly 24 variants of the AAT molecule have been identified, and all are inherited as codominant alleles. The most common M allele (PiM) may be found in 90% of people, and homozygous (PiMM) phenotypes produce serum levels within the reference range. The homozygous PiZZ state is the most common deficiency state and accounts for 95% of people in the severely deficient category.
Intravenous drug use
Emphysema occurs in approximately 2% of persons who use intravenous (IV) drugs. This is attributed to pulmonary vascular damage that results from the insoluble filler (eg, cornstarch, cotton fibers, cellulose, talc) contained in methadone or methylphenidate.
The bullous cysts found in association with IV use of cocaine or heroin occur predominantly in the upper lobes. In contrast, methadone and methylphenidate injections are associated with basilar and panacinar emphysema.
Immunodeficiency syndromes
Human immunodeficiency virus (HIV) infection has been found to be an independent risk factor for COPD, even after controlling for confounding variables such as smoking, IV drug use, race, and age. [21]
Apical and cortical bullous lung damage occurs in patients who have autoimmune deficiency syndrome and Pneumocystis carinii infection. Reversible pneumatoceles are observed in 10-20% of patients with this infection.
Vasculitis syndrome
Hypocomplementemic vasculitis urticaria syndrome (HVUS) may be associated with obstructive lung disease. Other manifestations include angioedema, nondeforming arthritis, sinusitis, conjunctivitis, and pericarditis.
Connective tissue disorders
Cutis laxa is a disorder of elastin that is characterized most prominently by the appearance of premature aging. The disease usually is congenital, with various forms of inheritance (ie, dominant, recessive). Precocious emphysema has been described in association with cutis laxa as early as the neonatal period or infancy. The pathogenesis of this disorder includes a defect in the synthesis of elastin or tropoelastin.
Marfan syndrome is an autosomal dominant inherited disease of type I collagen characterized by abnormal length of the extremities, subluxation of the lenses, and cardiovascular abnormality. Pulmonary abnormalities, including emphysema, have been described in approximately 10% of patients.
Ehlers-Danlos syndrome refers to a group of inherited connective tissue disorders with manifestations that include hyperextensibility of the skin and joints, easy bruisability, and pseudotumors; it has also been associated with a higher prevalence of COPD.
Salla disease
Salla disease is an autosomal recessive storage disorder described in Scandinavia; the disease is characterized by intralysosomal accumulation of sialic acid in various tissues. The most important clinical manifestations are severe intellectual disability, ataxia, and nystagmus. Precocious emphysema has been described and likely is secondary to impaired inhibitory activity of serum trypsin.
Epidemiology
The National Health Interview Survey reports the prevalence of emphysema at 18 cases per 1000 persons and chronic bronchitis at 34 cases per 1000 persons. [22] While the rate of emphysema has stayed largely unchanged since 2000, the rate of chronic bronchitis has decreased. Another study estimates a prevalence of 10.1% in the United States. [23] However, the exact prevalence of COPD in the United States is believed to be underestimated. This is largely due to the fact that it is an underdiagnosed (and undertreated) disease, because most patients do not present for medical care until the disease is in a late stage.
The exact prevalence of COPD worldwide is largely unknown, but estimates have varied from 7-19%. The Burden of Obstructive Lung Disease (BOLD) study found a global prevalence of 10.1%. [24] Men were found to have a pooled prevalence of 11.8% and women 8.5%. The numbers vary in different regions of the world. Cape Town, South Africa, has the highest prevalence, affecting 22.2% of men and 16.7% of women.
Hannover, Germany, on the other hand, has the lowest prevalence, of 8.6% for men and 3.7% for women. The differences can be explained in part by site and sex differences in the prevalence of smoking. As noted above, these reports are widely believed to be underestimates because COPD is known to be underdiagnosed and undertreated. Additionally, the prevalence in women is believed to be increasing.
Although current rates of COPD in men are higher than the rates in women, the rates in women have been increasing. COPD occurs predominantly in individuals older than age 40 years.
Severe, early onset disease likely represents a distinct genotype and is more commonly seen in females, African Americans, and those with a maternal family history of COPD. [25]
A study by Mintz et al estimated the prevalence of unidentified COPD. [26] Using the Lung Function Questionnaire (LFQ) and spirometry results, the study determined that approximately 1 in 5 patients (21%) aged 30 years or older with a history of smoking for 10 years or longer seen in a primary care center is likely to have COPD.
Prognosis
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, causing 3.23 million deaths in 2019. [1] In terms of COPD as the underlying cause of death, absolute mortality rates for US patients aged 25 years or older (2005) were 77.3 deaths per 100,000 males and 56.0 deaths per 100,000 females, or 64.3 persons per 100,000 overall. Internationally, overall mortality rates from COPD vary markedly, from more than 400 deaths per 100,000 males aged 65-74 years in Romania to fewer than 100 deaths per 100,000 population in Japan.
The FEV1 was used to predict outcome in COPD until other factors were identified to play a role in determining the outcome of COPD patients. These discoveries resulted in the creation of the multidimensional BODE index (body mass index, obstruction [FEV1], dyspnea [modified Medical Research Council dyspnea scale], and exercise capacity [6MWD]). [27] This index was developed to assess an individual’s risk of death or hospitalization.
Prognosis is based on a point system, with all 4 factors used to determine the score, as follows:
-
Body mass index: greater than 21 = 0 points; less than 21 = 1 point
-
FEV1 (postbronchodilator percent predicted): greater than 65% = 0 points; 50-64% = 1 point; 36-49% = 2 points; less than 35% = 3 points
-
Modified Medical Research Council (MMRC) dyspnea scale: MMRC 0 = dyspneic on strenuous exercise (0 points); MMRC 1 = dyspneic on walking a slight hill (0 points); MMRC 2 = dyspneic on walking level ground, must stop occasionally due to breathlessness (1 point); MMRC 3 = dyspneic after walking 100 yards or a few minutes (2 points); MMRC 4 = cannot leave house; dyspneic doing activities of daily living (3 points)
-
Six-minute walking distance: greater than 350 meters = 0 points; 250-349 meters = 1 point; 150-249 meters = 2 points; less than 149 meters = 3 points
The approximate 4-year survival based on the point system above is as follows:
-
0-2 points = 80%
-
3-4 points = 67%
-
5-6 points = 57%
-
7-10 points = 18%
The use of a clinical scoring system reinforces that determinants of prognosis in COPD remain multifactorial. Waschki et al argued that objective assessments of physical activity, including 6-minute walk test results, are best able to predict mortality. [28] However, additional socioeconomic factors also likely play a role in COPD prognosis; for example, a retrospective cohort study highlighted the increased risk of COPD-related mortality in patients who reside in isolated rural areas. [29]
A study by Sundh et al determined that the Clinical COPD Questionnaire (CCQ), which estimates quality of life in patients with COPD, is effective. [30] The CCQ identified that heart disease, depression, and underweight status are independently associated with lower health-related quality of life in patients with COPD.
In a multicenter, prospective, observational study of 201 consecutive patients with moderate-to-severe COPD, Martinez-Garcia et al reported that in addition to smoking, pulmonary hypertension, and declining lung function (known risk factors for mortality in patients with COPD), bronchiectasis (which is common in patients with moderate-to-severe COPD) is independently associated with increased risk of all-cause mortality. [31]
In this study, those who had bronchiectasis were found to be 2.5 times more likely to die than those who did not. Bronchiectasis remained an independent factor after adjustment for dyspnea, partial pressure of oxygen, body mass index, presence of potentially pathogenic microorganisms in sputum, presence of daily sputum production, number of severe exacerbations and peripheral albumin, and ultrasensitive C-reactive protein concentrations. [31]
Patient Education
It is important to educate the patient with COPD about the disease and to encourage his or her active participation in therapy. The 2 most essential points for the patient to understand are as follows:
-
The dangers of smoking and the improvement in quality of life attainable with smoking cessation
-
The need to seek medical care early during an exacerbation and to not wait until they are in distress
For patient education resources, visit the Lung Disease and Respiratory Health Center. Also, see the patient education articles Chronic Obstructive Pulmonary Disease (COPD), Smoking (Cigarette), Asthma, and Emphysema.
-
Venn diagram of chronic obstructive pulmonary disease (COPD). Chronic obstructive lung disease is a disorder in which subsets of patients may have dominant features of chronic bronchitis, emphysema, or asthma. The result is airflow obstruction that is not fully reversible.
-
Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells.
-
Histopathology of chronic bronchitis showing hyperplasia of mucous glands and infiltration of the airway wall with inflammatory cells (high-powered view).
-
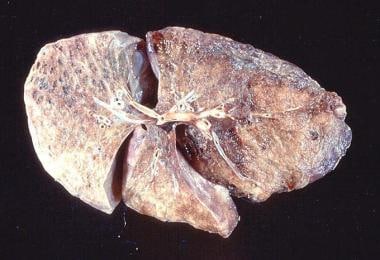
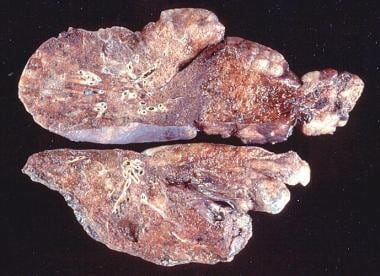
Gross pathology of advanced emphysema. Large bullae are present on the surface of the lung.
-
Gross pathology of a patient with emphysema showing bullae on the surface.
-
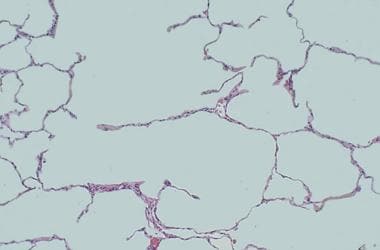
At high magnification, loss of alveolar walls and dilatation of airspaces in emphysema can be seen.
-
Posteroanterior (PA) and lateral chest radiograph in a patient with severe chronic obstructive pulmonary disease (COPD). Hyperinflation, depressed diaphragm, increased retrosternal space, and hypovascularity of lung parenchyma are demonstrated.
-
A lung with emphysema shows increased anteroposterior (AP) diameter, increased retrosternal airspace, and flattened diaphragm on lateral chest radiograph.
-
A lung with emphysema shows increased anteroposterior (AP) diameter, increased retrosternal airspace, and flattened diaphragm on posteroanterior chest radiograph.
-
Severe bullous disease as seen on a computed tomography (CT) scan in a patient with chronic obstructive pulmonary disease (COPD).
-
Pressure volume curve comparing lungs with emphysema, lungs with restrictive disease, and normal lungs.
-
Flow volume curve of a patient with emphysema shows marked decrease in expiratory flow, hyperinflation, and air trapping (patient B) compared with a patient with restrictive lung disease, who has reduced lung volumes and preserved flow (patient A).
-
Forced expiratory volume in 1 second (FEV1) can be used to evaluate the prognosis in patients with emphysema. The benefit of smoking cessation is shown here because the deterioration in lung function parallels that of a nonsmoker, even in late stages of the disease. Redrawn from Fletcher C, Peato R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645-1648.
-
Oxygen therapy via nasal cannula.
-
Home supplemental oxygen.
-
Bilevel positive airway pressure (BiPAP).
-
Pulmonary rehabilitation.
-
Chronic obstructive pulmonary disease (COPD). Pulmonary rehabilitation.
-
Chest radiograph of an emphysematous patient shows hyperinflated lungs with reduced vascular markings. Pulmonary hila are prominent, suggesting some degree of pulmonary hypertension (Correa da Silva, 2001).
-
Schematic representation of another sign of emphysema on the lateral chest radiograph. When the retrosternal space (defined as the space between the posterior border of the sternum and the anterior wall of the mediastinum) is larger than 2.5 cm, it is highly suggestive of overinflated lungs. This radiograph is from a patient with pectus carinatum, an important differential diagnosis to consider when this space is measured (Correa da Silva, 2001).
-
Close-up image shows emphysematous bullae in the left upper lobe. Note the subpleural, thin-walled, cystlike appearance (Correa da Silva, 2001).
-
A, Frontal posteroanterior (PA) chest radiograph shows no abnormality of the pulmonary vasculature, with normal intercostal spaces and a diaphragmatic dome between the 6th and 7th anterior ribs on both sides. B, Image in a patient with emphysema demonstrating reduced pulmonary vasculature resulting in hyperlucent lungs. The intercostal spaces are mildly enlarged, and the diaphragmatic domes are straightened and below the extremity of the seventh rib (Correa da Silva, 2001).
-
A, Lateral radiograph of the chest shows normal pulmonary vasculature, a retrosternal space within normal limits (< 2.5 cm), and a normal angle between the diaphragm and the anterior thoracic wall. B, Lateral view of the chest shows increased pulmonary transparency, increased retrosternal space (>2.5 cm), and an angle between the thoracic wall and the diaphragm >90 degrees. Straightening of the diaphragm can be more evident in this projection than on others (Correa da Silva, 2001).
-
High-resolution CT (HRCT) in a patient after viral bronchiolitis obliterans demonstrates areas of airtrapping, which is predominant in the inferior lobes and associated with bronchiectasis in the left lower lobe. Note that the decreased attenuation caused by the airtrapping can simulate emphysema (Correa da Silva, 2001).
-
Pediatric high-resolution CT (HRCT) shows a hyperinflated right lung with large pulmonary bullae due to congenital lobar emphysema (Correa da Silva, 2001).
-
High-resolution CT (HRCT) demonstrates areas of centriacinar emphysema. Note the low attenuation areas without walls due to destruction of the alveoli septae centrally in the acini. Red element shows the size of a normal acinus (Correa da Silva, 2001).
-
High-resolution CT (HRCT) shows large bullae in both inferior lobes due to uniform enlargement and destruction of the alveoli walls causing distortion of the pulmonary architecture (Correa da Silva, 2001).
-
Panacinar emphysema of the left lung in a patient with a right lung transplant. Note the red element showing the size of a normal acinus and its discrepancy with the destroyed and enlarged airspaces of the left lower lobe (Correa da Silva, 2001).
-
High-resolution CT (HRCT) shows subpleural bullae consistent with paraseptal emphysema. Red mark shows the size of a normal acinus (Correa da Silva, 2001).
-
High-resolution CT (HRCT) shows enlarged air-spaces or bullae adjoining pulmonary scars, consistent with paracicatricial emphysema. Red mark shows the size of a normal acinus (Correa da Silva, 2001).
-
CT densitovolumetry of a nonsmoker, healthy young patient shows normal lungs. Less than 0.35% of lungs have attenuations below -950 HU (Correa da Silva, 2001).
-
Expiratory CT densitovolumetry shows no areas of airtrapping (Correa da Silva, 2001).
-
CT densitovolumetry in a patient with lung cancer. Three-dimensional (3D) image shows that the cancer is in the portion of the right lung that was less affected by emphysema in a patient with poor pulmonary function (Correa da Silva, 2001).
-
CT densitovolumetry shows the attenuation mask. Green areas are those with attenuation below the selected threshold (here, -950 HU to evaluate emphysema), and pink areas are those with attenuations above the threshold. Area outside the patient is highlighted in green because of air (Correa da Silva, 2001).
-
CT densitovolumetry demonstrates irregular distribution of the emphysema, with substantial predominance in the left lung (Correa da Silva, 2001).
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Smoking Cessation
- Management of Inflammation
- Management of Infection
- Management of Sputum Viscosity and Secretion Clearance
- PPIs for Exacerbations and the Common Cold
- Oxygen Therapy and Hypoxemia
- Vaccination to Reduce Infections
- Alpha1-Antitrypsin Deficiency Treatment
- Inpatient Care
- Bullectomy
- Lung Volume Reduction Surgery
- Lung Transplantation
- Long-term Monitoring
- End-of-Life Care
- Show All
- Guidelines
- Medication
- Medication Summary
- Beta2-Adrenergic Agonists, Short-Acting
- Beta2-Adrenergic Agonists, Long-Acting
- Anticholinergics, Respiratory
- Xanthine Derivative
- Phosphodiesterase-4 Inhibitors
- Dual PDE-3/PDE-4 Inhibitors
- Corticosteroids, Inhalant
- Corticosteroids, Oral
- Beta-Adrenergic Agonist and Anticholinergic Agent Combinations
- Beta2-Adrenergic Agonist and Corticosteroid Combinations
- Other Combinations
- Antibiotics
- Smoking Cessation Therapies
- Interleukin Inhibitors
- Show All
- Media Gallery
- References