Practice Essentials
A polyp is a small growth of tissue shaped like the head or stalk of a mushroom. Two types of polyps develop in the wall of the colon: hyperplastic (harmless) and adenomatous (precursor to cancer). An adenomatous polyp 10 mm in diameter takes 5-10 years to become dangerous, leaving a large window of opportunity in which to find and remove it.
There are several colorectal screening options for average-risk individuals to detect colonic polyps, including colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, double-contrast barium enema every 5 years, CT colonography every 5 years, and annual fecal occult blood testing. Colonoscopy is the gold standard for screening because it allows for both detection and excision of premalignant lesions. [1, 2, 3, 4]
Unlike colonoscopy, CT colonography does not require sedation. In a number of studies, CT colonography has displayed results equivalent to colonoscopy in both cancer and polyp detection. CT colonography has been shown to rapidly and effectively examine the entire colon for lesions. Emerging indications for CT colonography may include evaluation of patients with sigmoid colonic stoma and those affected by deep pelvic endometriosis and could be useful in chronic diverticular disease to differentiate between inflammatory and neoplastic stenoses. [5, 6, 7, 8, 9, 10]
Efforts are underway to find all polyps 5-10 mm in diameter using computed tomography (CT) scanning or magnetic resonance imaging (MRI). [11, 12] These modalities are employed in the evolving techniques for so-called computed colonography (also called virtual colonography or virtual colonoscopy) examinations. Electronic chromoendoscopy is an approach that includes narrow band imaging. [13]
The impact of computed colonography on health-care costs depends on the component costs and the percentage of patients that go on to intervention. For example, if computed colonography provides high confidence that no lesions are present, it saves the difference in cost between it and colonoscopy. However, if a computed colonography examination finds a lesion that merits treatment by colonoscopic polypectomy, then computed colonography represents an added cost. Thus, computed colonography offers the most financial benefit when the ratio of its cost to colonoscopy is lower than the incidence of negative cases. In practice, computed colonography can lower costs, but not at all centers. [14]
Other tests, including stool heme testing, sigmoidoscopy, and double-contrast barium enema (DCBE) study, are less accurate; with these tests, between one third and one half of all treatable lesions can be missed. (As many as 55% of precancerous lesions can be missed with sigmoidoscopy.)
Of patients tested for heme in the stool, 1 in 5 has a positive result, and of those who test positive, one third have colonic polyps and 1 in 20 have colon cancer. Among individuals with negative heme results, approximately 1 in 30 has colon cancer. Conversely, of patients with colon cancer, 50% have negative results on stool heme tests. (In fact, more than 90% of known colonic lesions test negative for occult blood, because polyps do not cause bleeding unless they are torn as a result of a shear force. Moreover, cancerous lesions do not commonly bleed.) Careful analysis of statistical data indicates that the primary benefit of these tests is to motivate patients to comply with recommended optical colonoscopy examinations.
The fecal immunochemical test (FIT) may be an alternative. FIT detects globin rather than heme, making it more specific for human blood. FIT is replacing heme stool testing in screening programs because of its ease of use, increased uptake, quantitative analysis, and greater sensitivity for advanced colorectal neoplasia (ACN). Sensitivity estimates are almost 90%. [15]
Halligan argues that CT colonography (CTC) to investigate patients with symptoms potentially suggestive of colorectal cancer should replace barium enema, because CTC is a sensitive, acceptable, and equally cost-effective alternative to colonoscopy in patients in whom colonoscopy is contraindicated or undesirable. This rationale is based on the findings of the UK Special Interest Group in Gastrointestinal and Abdominal Radiology (SIGGAR) randomized controlled trials, which compared CTC with barium enema or colonoscopy for diagnosis of colorectal cancer or large polyps in symptomatic patients. Diagnostic outcomes looked at both intracolonic and extracolonic disease, along with psychological reactions of patients to the tests and cost-effectiveness of the different diagnostic strategies. [16] The study in The Lancet concludes that based on a randomized trial of 3838 patients, CTC is a more sensitive test than barium enema and is recommended as the preferred radiologic test for patients with symptoms suggestive of colorectal cancer. [17]
A systematic review and meta-analysis of post-CT colonography by Obaro et al looked at interval or post-imaging colorectal cancer rates. The investigators concluded that CT colonography did not lead to an excess of post-test cancers relative to colonoscopy within 3-5 years. These results suggest that the recommended screening interval of 5 years is safe. [18]
The use of CT colonography for surveillance after colorectal cancer resection has also been suggested to lesson the need for endoscopic services. Among the cited benefits is that unlike colonoscopy, diagnostic contrast-enhanced CT colonography effectively evaluates both the intra- and extra-luminal aspects of the anastomosis. [19]
(See the images of colon polyps below.)
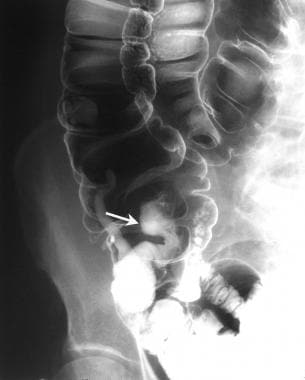
 Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
For individuals at average risk, the American Cancer Society recommends that an optical colonoscopy be performed every 5 years, starting at age 50 years, to remove polyps 5-10 mm in size. [20, 21] This practice reduces the incidence of colon cancer more than 90%. [22] However, compliance with this recommended procedure is only 40%; therefore, efforts to develop reliable alternative tests are continuing.
Although nuclear medicine has no role in the diagnosis of colonic polyps, associated abnormalities in some of the polyposis syndromes lend themselves to radionuclide imaging. One example is Gardner syndrome, in which the osseous lesions, thyroid cancer, and small-bowel carcinoids can be imaged with radionuclides. [23]
There are new endoscopic imaging technologies being studied that promise to generate images of the cellular structure of polyps. These are known as optical biopsy or virtual histology and include confocal laser endomicroscopy, optical coherence tomography, multiphoton microscopy, Raman spectroscopy, hyperspectral imaging, and molecular imaging. [24]
Limitations of techniques
The false-positive rate for stool heme testing is high, as is the false-negative rate. The stool heme test in patients with known colon cancer commonly provides negative results. Studies have shows a benefit in one third of those patients who undergo a stool heme test who are subsequently referred for colonoscopy, and the net reduction in mortality rates is one third when everyone is referred; therefore, stool heme testing is not effective. [25, 26, 27, 28]
Sigmoidoscopy and barium enema study do not permit examination off the right side of the colon, where an increasing percentage of lesions occur. A colonoscopy, performed every 5 years starting at the age of 50 years (or 40 years in patients with risk factors, such as a family history), is the best screening method.
Failure to diagnose cancer is a leading cause of malpractice litigation against gastroenterologists. [29, 30]
A study in which different examiners performed 2 consecutive colonoscopy procedures on the same day revealed a miss rate of 24% for adenomas overall and a miss rate of 6% for adenomas of 1 cm or more. [29, 30, 31] Of the misses on colonoscopy, one half are ascribed to perception, and one half are ascribed to incomplete viewing.
Optical colonoscopy cannot be used to visualize areas behind tissue folds or to look through retained feces, and the tip of the scope may not reach the end of the colon. [32]
Retrospective data support the conclusion of a miss rate greater than 5%. Of 941 patients with colon cancer who underwent colonoscopy within the preceding 3 years, 5% of the results were interpreted as negative even though dangerous cancer precursors must have been present. Because most lesions do not progress to cancer, the miss rate for lesions is considerably higher. Nevertheless, colonoscopy compares favorably with DCBE study, which had a miss rate of 17% in 719 of patients who underwent barium enema study within the previous 3 years. Most of the difference between colonoscopy and barium enema study is in the ability to detect early, curable colon cancers and precursor lesions.
Interventional optical colonoscopy
The primary interventional procedure for the evaluation and removal of colonic polyps is optical colonoscopy, and gastroenterologists typically perform this procedure. Optical colonoscopy involves preparation to evacuate and clean the colon, followed by the passage of the long, fiberoptic colonoscopic tubing from the anus to the ileocecal valve. During this passage, all surfaces should be viewed en route or when the tubing is pulled back, through the video-enabled, distal tip of the flexible tube.
Air and saline are injected, the scope is rotated, and the tip is flexed or extended by using cables. The patient is rolled into different positions as needed. Conscious sedation is used to reduce spasm of the colon and to control pain. Polyps are removed by using a snare threaded to the distal end of the device.
Guidelines
The U.S. Multi-Society Task Force on Colorectal Cancer (MSTF) guidelines for colorectal cancer screening include the following [33, 34] :
-
Clinicians should offer screening for colorectal cancer beginning at age 50 years for non-black average-risk individuals (beginning at age 45 years for black individuals).
-
Colonoscopy is recommended every 10 years or an annual fecal immunochemical testing (FIT) for those at average risk for colorectal neoplasia.
-
Physicians performing screening colonoscopy should measure quality, including the adenoma detection rate.
-
CT colonography is recommended every 5 years or FIT-fecal DNA every 3 years or flexible sigmoidoscopy every 5-10 years in those who refuse colonoscopy and FIT.
-
Clinicians should offer annual FIT to those with one or more first-degree relatives with colorectal cancer or documented advanced adenomas but who decline colonoscopy.
-
It is recommended that adults younger than 50 years with colorectal bleeding symptoms undergo colonoscopy or an evaluation sufficient to identify the cause of bleeding, and that clinicians initiate treatment and thorough follow-up to determine resolution of the bleeding.
Radiography
The principal radiographic method for assessing colonic polyps is double-contrast barium enema (DCBE) study, which requires a good deal of experience to perform well. Barium enemas served a useful purpose in the past, but enthusiasm for DCBE studies has declined because of their low sensitivity to polyps smaller than 1 cm in diameter. These studies have also become less popular because they have difficulty detecting polyps in areas in which a single lumen is not detectable because of overlap or redundancy (eg, sigmoid, rectosigmoid, hepatic, and splenic flexures). [35]
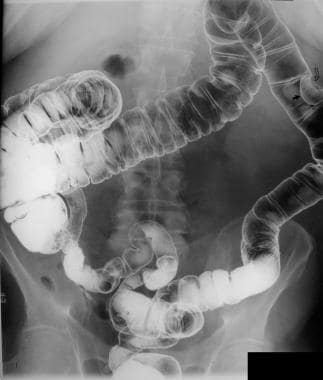
When a single lumen is not detectable (and a colonoscopy cannot be performed), the problem is offset by combining a DCBE study with flexible sigmoidoscopy. However, even this combination of tests is considered inadequate for the surveillance of familial polyposis, familial colon cancer, and inflammatory bowel disease, in which an attention to small details of the colonic mucosa is required and the likelihood of biopsy or polyp removal is high (see the images below).
 Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
Sigmoidoscopy does not permit imaging in more than one third of the colon, and the prevalence of lesions beyond the reach of sigmoidoscopy has been increasing. Optical colonoscopy is preferred over DCBE study and sigmoidoscopy. Barium enema study causes too many treatable lesions to be missed, and in one multicenter trial, its sensitivity was as low as 43% for 1-cm polyps. [35]
The radiographic detection of colonic polyps depends on the size of the lesion present and the type of examination employed. For polyps 1 cm or larger, the sensitivity of DCBE and single-contrast barium enema studies is reported to be 90-95% (which is similar to the sensitivity of colonoscopy in detecting these lesions), although data from Winawer and colleagues suggest that this rate is high. [36, 37, 38] DCBE is more sensitive in the detection of polyps smaller than 1 cm.
Compared with physician review of computed colonography data, automated computer detection of lesions can perform well for larger polyps (90% for medium-sized polyps [6-9 mm] and 86% for large polyps [10+ mm], as opposed to 51% for smaller polyps), with a per-patient sensitivity of 73% for automated detection versus 89% for radiologist review. Excluding polyps more than 5 mm in diameter, the per-patient sensitivity has been reported to be 100% for the radiologist and 96% for automated analysis. [39]
Because of the appearances of polyposis syndromes in the colon, there is often little doubt regarding the nature of the lesions demonstrated on contrast-enhanced examination in a well-prepared bowel, as seen in optimal double contrast. The features of a multiplicity of polyps in a young person with extracolonic manifestations of disease aid in the diagnosis.
The false-positive rate for DCBE study is less than 5% in patients between the ages of 50 and 75 years receiving annual fecal occult blood tests. However, these patients have a high probability of receiving a false-positive result. The false-negative rate for the detection of polyps smaller than 1 cm is 7% on DCBE testing.
Computed Tomography
The European Society of Gastrointestinal Endoscopy (ESGE)/European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guidelines recommend the use of CT colonography as the radiologic examination of choice for diagnosis of colorectal neoplasia, in the case of incomplete colonoscopy, and as an acceptable and equally sensitive alternative for patients with symptoms suggestive of colorectal cancer, when colonoscopy is contraindicated or not possible. However, the ESGE-ESGAR guideline does not recommend CT colonography for population screening. [10] CT colonography (CTC) images the entire colon, using a helical and, preferably, multidetector-row volumetric CT scanner. Contiguous sections with 1- to 3-mm collimation are acquired in a single breath-hold. [40, 41, 42, 5, 6, 7, 8, 9]
Colonoscopy is the reference standard for the detection of colorectal cancer, but it is invasive and has the associated risks of bowel perforation and bleeding. Unlike colonoscopy, CT colonography does not require sedation. In a number of studies, CT colonography has displayed results equivalent to colonoscopy in both cancer and polyp detection. CT colonography has been shown to rapidly and effectively examine the entire colon for lesions. Emerging indications for CT colonography may include evaluation of patients with sigmoid colonic stoma and those affected by deep pelvic endometriosis and could be useful in chronic diverticular disease to differentiate between inflammatory and neoplastic stenoses. [5, 6, 7, 8, 9, 10]
Preparation and technique
Bowel preparation ideally consists of full colon cleansing using relatively dry preparation, such as that employing phosphosoda or magnesium citrate. These cathartics leave less residual fluid than does the colonic lavage typically used for optical colonoscopy. The residual fluid is not desirable because it can obscure polyps and masses. Alternatively, a low-fiber diet may be combined with milder preparation (eg, with bisacodyl tablets and magnesium citrate) and with stool tagging with barium and/or a water-soluble contrast agent. The barium helps by tagging residual stool, and the water-soluble contrast agent helps by tagging the residual fluid. Tagging of stool with barium is probably sufficient, unless software that electronically subtracts the high attenuation residual fluid is used; in that case, barium alone is insufficient. [43, 44, 45]
Optimal distention of the colon is critical to produce an interpretable data set. Glucagon or Buscopan is sometimes administered just before the examination to make the patient more comfortable, but its effect on polyp detection is controversial. Distention can be accomplished with mechanical or manual techniques. Manual insufflation by using a "blue puffer" can be performed by the technologists or by the patients themselves (self-insufflation); for this, room air or carbon dioxide can be used.
One commercially available mechanical pump was designed specifically for insufflation of the colon with carbon dioxide, for use with CTC. It starts insufflating slowly, for patient comfort, and then inflates at a set rate until a pressure of 25 mm Hg is reached. Use of such a pump permits the trained technologist to perform the entire examination without the need for assistants. Also, carbon dioxide is more comfortable than air because carbon dioxide is released by means of blood absorption and breathing.
Retained fluid may obscure some colon surfaces, but this problem may be resolved by improving preparation, by imaging twice (for example, with the patient in the supine and prone positions), or by using an oral or intravenous contrast agent. Acquisition and review of supine and prone CT colonography images significantly improves the ability to identify patients with polyps that are 0.5 cm or larger in diameter. The administration of oral iodinated contrast medium does not significantly improve polyp detection. If a large volume of retained fluid is seen on the supine view, the prone view may be acquired with the administration of an intravenous contrast agent to help identify lesions. Alternatively, an additional decubitus view may be obtained to shift the position of the fluid.
Scanning should be done by using a collimation of 3 mm or less, a reconstruction interval one half to one third of the collimation, a soft or standard algorithm, a single breath-hold of 9-20 seconds (depending on the capabilities of the scanner), and a relatively low mAs setting. Regarding this last parameter, a 30-100 mAs setting has proven to give diagnostic results, with larger patients requiring a setting of 80-100 mAs.
Two-dimensional (2D), cross-sectional images may be examined in conjunction with multiplanar reconstructions (sagittal, coronal, or oblique views) and 3-dimensional (3D) endoluminal views (surface or volume rendered). Regardless of whether one views the 2D images first (a primary 2D reading) or the 3D images first (a primary 3D reading), both views are complementary. Experimentally, the current authors have found advantages to adding surface-volume projections and virtual holograms. Volumetric reconstruction methods appear to improve sensitivity and specificity. With multidetector-row CT scanning, submillimeter section thicknesses are possible and allow detailed reconstruction of the images on a workstation, in a multiplanar format. [46]
Experience
The interpretation of computed colonography images requires training and practice. At one of the authors' institutions, virtual holography had 100% sensitivity and specificity in detecting 40 polyps of 2-5 mm in size in a porcine model of colonic polyposis (translucent, parallel holography system designed and developed by Dr. Pearlman).
Surface reconstruction of images can show the colonic wall in a manner similar to that seen with optical endoscopy of the colon. [47] The triangular appearance is characteristic of folds in the transverse colon; hence, some practitioners call these methods virtual colonoscopy. However, if a threshold is applied to compute the apparent surface, the computed surface may not correspond to an actual surface. False polyps, bridges, ulcers, recesses, and other convincing artifacts can be created or destroyed by a small change in the parameters used to construct the images. To solve that problem, one of the authors developed a method of simulated holography, which is a form of translucent volume rendering (see the images below).
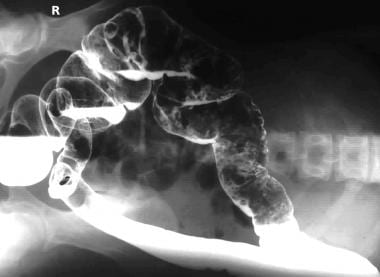
 Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
 Surface reconstruction of images can identify the colonic wall in a manner that appears like optical endoscopy of the colon. The triangular appearance is characteristic of folds in the transverse colon; hence, some practitioners term these methods virtual colonoscopy.
Surface reconstruction of images can identify the colonic wall in a manner that appears like optical endoscopy of the colon. The triangular appearance is characteristic of folds in the transverse colon; hence, some practitioners term these methods virtual colonoscopy.
Experience is required to learn how to detect polyps and distinguish diverticula, stool, flexural pseudotumors, and other pitfalls. A sufficient number of abnormal, endoscopically proven cases should be interpreted before one ventures into virtual colonoscopy. This experience can often be gained in collaboration with supportive gastroenterologists, who permit recruitment of above-average risk patients who are already scheduled for optical colonoscopy for CTC (approved for institutional review board) done on the same day before optical colonoscopy.
Technology
3D virtual colonoscopy techniques have the appeal of truly simulating conventional colonoscopy. Several commercial workstations are incorporating a center path line that allows the computer to automatically generate images and make cine images of the colon traversing this center path. With improvements in the software, one can then navigate through the colon and evaluate suspicious abnormalities.
Several investigators (including the author) pioneered advanced computer-aided detection (CAD) software for CTC, and vendors of CT have produced and released different versions. Analogous to its use in mammography, CAD can serve as a "spell checker" to help find the polyps. Various techniques are used to reduce the number of false-positive results by teaching the CAD software to recognize stool (eg, on the basis of its average attenuation and its attenuation gradient). Some CAD programs reportedly have a sensitivity of >90% for 10-mm polyps, with only 1-2 false-positive findings per case. CAD may be critical in increasing the confidence of interpretation for inexperienced CTC readers and in facilitating the use of CTC on a routine basis in the mainstream radiology community. [48, 49]
Software programs dedicated to CTC have rapidly proliferated, and improvements are continually being implemented. Ideally, CTC software should have a user-friendly interface that allows for the rapid comparison of axial, multiplanar reconstruction (MPR) and 3D images. Other features should be a fast, semi-automated fly-through image of the endoluminal view, easy comparison of supine and prone images, easy comparison of lung and soft-tissue windows, reliable means to eliminate artifacts (false contours), and reliable means to locate lesions and make measurements. Surface or volume-rendered images produce excellent endoluminal views, but they sometimes produce false contours (isointensity threshold is not necessarily a true surface). Virtual holography avoids that problem.
Some software packages have novel views that virtually cut the colon open or simultaneously display forward, sideways, and backwards views of the lumen. These views may help to reduce reading time, because only 85% of the mucosal surface is seen on a unidirectional fly-through image. Therefore, to see all of the mucosa, forward and backwards fly-through views are necessary on the supine and prone images.
Fluid and stool opacification combined with electronically subtracted images are most amenable to a primary 3D interpretation. Most experts agree that a classic 2D reading is needed in an intermediate (wide) soft-tissue window to detect flat or infiltrating lesions. Lesion size should be carefully measured on 2D images; however, oval or irregularly shaped lesions are sometimes more accurately measured on 3D images.
Sensitivity/Specificity
A wide range of data is reported. Sosna and colleagues performed a meta-analysis and found that, at the 10-mm threshold, by-patient sensitivity was 88% and by-polyp sensitivity was 81%. At the 5-9.9 mm threshold, the by-patient sensitivity was 84%, and the by-polyp sensitivity was 62%.
Later results in a few large cohorts were similar, worse, or better. In all cohorts, the specificity is usually 90-95%. The best results were those that Pickhardt and colleagues achieved; they had a 92% by-polyp sensitivity for adenomas 8 mm or larger. [19]
The American College of Radiology Imaging Network (ACRIN) enrolled more than 2,500 patients in 15 centers to assess the effectiveness of virtual colonoscopy, with a report of a per-patient sensitivity of 90% for adenomas 1 cm and larger and a per-adenoma sensitivity of 84% for polyps 1 cm and larger. [50]
The incidence of polyps in patients older than 40 years is sufficiently high that the most important factor in any test for polyps, other than optical colonoscopy, is the true-negative rate. If CT scanning can show true-negative results with high confidence, patients may be spared the pain, discomfort, embarrassment, and risk of optical colonoscopy for an additional 5 years. Patients with positive findings gain little, because they must undergo optical colonoscopy for lesion removal. For these patients, CTC may be a waste of time and money. However, if the cost of CTC is small compared with the cost of optical colonoscopy, then CTC is cost effective if the ratio of CTC to optical colonoscopy is less than the fraction of the population in which CTC shows negative findings.
Limitations
CTC may improve compliance with the necessary screening. It can help those who perform optical colonoscopy by increasing compliance with referrals and by increasing the fraction of examined patients who require treatment. The observer must confirm that the entire colonic mucosal surface is viewed and that the viewing parameters do not produce significant artifact, which affects sensitivity.
Adequate bowel preparation is absolutely vital for the confident detection of significant lesions, because residual fecal material may be indistinguishable from polyps or neoplasms. It may also obscure polyps, making their detection impossible.
Other problems in the detection of polyps with CTC arise with small (5- to 9.9-mm), sessile polyps. Distinguishing polyps from haustral folds also can be difficult; therefore, a clean, well-distended colon must be visualized with the patient in the prone and supine positions.
With workstations incorporating a center path line, problems are encountered when segments of the colon are not well distended and when the centerline cannot be generated. Also, in overdistended bowel segments, the centerline may jump to an adjacent distended loop. Problems with the workstation may also occur with a 3D-viewing facility, so that the entire colonic surface is not always evaluated.
Further limitations of primary 2D and primary 3D techniques are that they cannot be used as the sole technique for image interpretation. Use of the 3D technique alone may result in many false-positive results. Just as 3D imaging and MPR are used for problem solving when 2D imaging is the primary interpretation technique, 2D imaging must be used as a problem-solving method for 3D imaging. This approach helps in evaluating the attenuation characteristics of a lesion and in determining if a filling defect is a mural abnormality or an extrinsic defect.
Often, lesions are detected by using 3D CT colonography techniques demonstrating morphology that is consistent with that of a polyp or neoplasm. When these same areas are investigated with 2D CT scanning, a variety of normal structures, including fecal material and extrinsic defects, may be found.
Finally, as stated above, the amount of time needed to evaluate the data with these 3D techniques may limit their usefulness in a clinical setting. Primary 3D imaging techniques must become quicker and more automated, with easier navigation, before they can become primary viewing techniques. However, given these limitations, polyps smaller than 5 mm can be routinely detected by using 3D endoluminal imaging performed in antegrade and retrograde fashion.
One study involved the use of axial images, as well as complete 3D endoluminal navigation in antegrade and retrograde directions with the patient in supine and prone positions. The detection of polyps smaller than 5 mm was 59%. This rate favorably compares with those of a second report in which only 2D imaging was used as the primary data-interpretation technique. Another study showed that 68% of polyps 5 mm or smaller that were missed with a primary 2D technique were either hyperplastic or normal colon, as determined at pathology.
Magnetic Resonance Imaging
MRI can depict the colon on contiguous sections 5 mm or thinner obtained with fast T1- or T2-weighted imaging sequences and a 512 × 512 matrix. Not all MRI systems can acquire images fast enough at that matrix size. [51] As with CT, dilating the emptied colon—for instance, with air or carbon dioxide—is important.
Gadopentetate dimeglumine may be given intravenously or intraluminally. Intravenous contrast enhancement may help in identifying dangerous lesions. Nonabsorbed, iron-particulate oral contrast agents are available; these may improve the resolution of the bowel and improve lesion detection.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography (MRA) scans. Characteristics of NSF/NFD include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Fecal tagging with magnetic resonance (MR) colonography without colonic cleansing has been used successfully with the oral administration of gadoterate meglumine (gadolinium-DOTA [Gd-DOTA]). The rigors often associated with colonic cleansing can affect the patient's willingness to accept large bowel imaging. Large bowel preparation could be eliminated if stool acquires a signal intensity different from that of colonic polyps. In theory, this goal can be achieved by showing dark polyps surrounded by bright fecal matter and a bright enema or bright polyps surrounded by dark fecal matter and a dark enema. The first approach can be achieved by using oral Gd-DOTA. [52]
Data from MRI may be viewed as 2D sections, surface renderings, or derivatives of volumetric or holographic views. Compared with CT scanning, MRI generally has an advantage in that it improves soft-tissue contrast by 10-fold or more. However, in this application, the limits of resolution and speed are tested, and the results of CT and MRI are comparable. Regardless, MRI has the advantage of a lack of ionizing radiation and its better-tolerated intravenous contrast agents.
As with CT scanning, virtual MR colonoscopy does not replace colonoscopy in demonstrating small colonic polyps. Overall experience with the technique is limited. However, the lack of adverse affects and of ionizing radiation warrant further consideration of the use of MR colonography to screen for gastrointestinal polyps. The limited experience so far suggests that polyps as small as 6 mm can be assessed, as can the inner wall.
Contrast agent opacification and inadequate colonic distension, as well as air bubbles and fecal masses, may present potential problems in the interpretation of the images.
Ultrasonography
Ultrasonography is not used to evaluate polyps because the air-water interfaces attenuate more than 90% of the ultrasonographic signal. In theory, ultrasonography can potentiate optical evaluations by enabling a view through the fluid or into the colon wall.
Ultrasonography is insensitive in the diagnosis of colonic polyps. However, bowel wall thickening may be seen with malignant transformation when the bowel wall is infiltrated. If the bowel wall is thickened, compressed, or obliterated, a carcinoma should be suspected.
A target or an atypical target sign is generally seen with an asymmetrical, hypoechoic thickening of the bowel wall in association with a central echogenic area due to the presence of intraluminal air and mucus.
The colon may be ultrasonographically examined for polyps or colon carcinoma by means of the retrograde instillation of fluid (generally, a warm sodium chloride solution) into the colon. By using a warm, isotonic sodium chloride enema, visualization of the rectosigmoid may be improved. A real-time examination of the colon can be performed with the sodium chloride technique.
Colonic polyps appear as hyperechoic structures projecting into the lumen of the colon. Endorectal ultrasonography can be used in the assessment of rectal carcinoma.
With the sodium chloride enema technique, colonic polyps larger than 7 mm can be identified in 91% of patients. However, at present, ultrasonography cannot replace a barium enema study or colonoscopy for the detection of colonic polyps. Still, an ultrasonographic examination is an invaluable tool for the screening of patients with polyposis syndromes and for the screening of their families for associated cancers, such as those of the thyroid, breast, liver, ovaries, and uterus.
Colonic polyps may be difficult to identify on ultrasonograms obtained with the patient in the supine position, because air normally collects anteriorly, causing distal acoustic shadowing that obscures the field. Moreover, the presence of feces and mucus also prevents the identification of colonic polyps. Adequate assessment of the bowel thickness at the base of the colonic polyp may not be possible by using the saline enema technique.
Neuroendocrine tumors (NETs) of the colon and rectum are rare, but their identification is important, as their management is different. Colonic and rectal NETs is recognized with greater frequency because of the wide spread use of endoscopy and improved histological reporting. Classification with the World Health Organization tumor, node, metastasis (TNM) staging system, as well as grading based on the Ki-67 index, has led to improved prognostic assessment. The use of endoanal ultrasound has increased the sensitivity of detection of depth of invasion and lymphovascular involvement, which is associated with a poor prognosis. [53]
-
Image shows a large, stalked polyp in the cecum. Histologically, the polyp was hamartomatous.
-
Double-contrast enema study in a 58-year-old man shows a solitary polyp with malignant change.
-
Left lateral decubitus image obtained as a part of a barium enema study shows multiple polyps in the transverse and descending colon.
-
Surface reconstruction of images can identify the colonic wall in a manner that appears like optical endoscopy of the colon. The triangular appearance is characteristic of folds in the transverse colon; hence, some practitioners term these methods virtual colonoscopy.
-
Using simulated holography to avoid artifacts, the author and colleagues achieved greater than 95% sensitivity and specificity in finding lesions larger than 5 mm in diameter, such as the lesion shown here. The manner of image reconstruction (custom software, orthogonal holographic projection) enables direct measurement of the lesion diameter along the line of measurement.