Practice Essentials
Neuropathic arthropathy (Charcot joint) can be defined as bone and joint changes that occur secondary to loss of sensation and is most often associated with diabetes, [1] syphilis, syringomyelia, [2] spina bifida, traumatic spinal cord injury, [3] and leprosy. [4] The radiographic changes of this condition include destruction of articular surfaces, opaque subchondral bones, joint debris, deformity, and dislocation (see the images below). Neuropathic arthropathy (Charcot joint) poses a special problem in imaging when it is associated with a soft-tissue infection. [5, 6]
Leprosy is one of many causes of neuropathic arthropathy; joint manifestations of this disease include signs of Charcot disease, which advances despite treatment. Neuropathic arthropathy related to diabetes, syphilis, leprosy, and connective tissue disorders is more common in the elderly population. Neuroarthropathy related to asymbolia, spina bifida, and spinal trauma is more common in young individuals. Sensory impairment associated with spina bifida and myelomeningocele is the most frequent cause of neuropathic arthropathy (Charcot joint) in childhood.
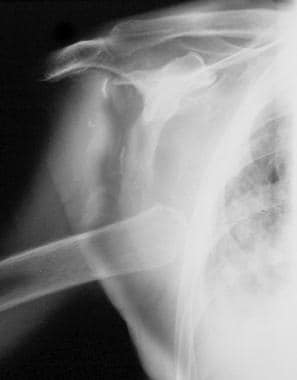 Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy of the shoulder in a patient with syringomyelia. Note the destruction of the articular surface, dislocation, and debris, which are pathognomonic for a neuropathic joint.
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy of the shoulder in a patient with syringomyelia. Note the destruction of the articular surface, dislocation, and debris, which are pathognomonic for a neuropathic joint.
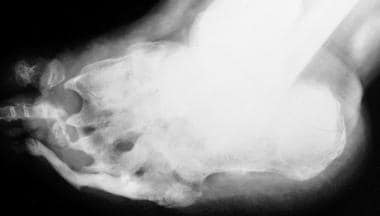 Neuropathic arthropathy (Charcot joint). Osteolysis of the distal metatarsals and phalanges with tapering results in a pencil-like appearance in the late stage of diabetic neuropathy.
Neuropathic arthropathy (Charcot joint). Osteolysis of the distal metatarsals and phalanges with tapering results in a pencil-like appearance in the late stage of diabetic neuropathy.
Charcot arthropathy can occur at any joint; however, it occurs most commonly in the lower extremity, at the foot and ankle in patients with diabetes. It is seen in up to 9% of patients with diabetic neuropathy. [1] Similar pathology can also occur in the knee but is much less common and should be considered in a patient with peripheral neuropathy, who presents with a red, hot, tender, and swollen knee joint. [7]
Syringomyelia is the main cause of upper extremity Charcot arthropathy, most often in the shoulder, although wrist and interphalangeal joint involvement have also been reported. [2]
Charcot spinal arthropathy (CSA), also called spinal neuroarthropathy, is a rare mechanical destructive process that affects the intervertebral disc and adjacent vertebral bodies in patients who have loss of joint protective mechanisms. [3]
Neuropathic arthropathy can be classified into hypertrophic and atrophic types. Hypertrophic changes predominate in the upper motor neuron lesions, and atrophic changes occur in peripheral nerve injuries. The early stage of osteoarthritis simulates neuropathic arthropathy, both radiologically and pathologically.
Progressive joint effusion, fracture, fragmentation, and subluxation should raise the suspicion of neuroarthropathy. In the advanced stage, abnormal findings on radiographs include subchondral sclerosis, osteophytosis, subluxation, and soft-tissue swelling. Long-standing neuroarthropathy is characterized by disorganization of joints. The finding of considerable amounts of cartilaginous and osseous debris within the synovial membrane (termed detritic synovitis) should alert the pathologist that the changes may represent a neuropathic joint. Other causes of detritic synovitis include osteonecrosis, calcium pyrophosphate dihydrate crystal deposition disease, psoriatic arthritis, osteoarthritis, and osteolysis with detritic synovitis.
Preferred examination
Radiography may be the only imaging required for the diagnosis of neuropathic arthropathy (Charcot joint). In the appropriate clinical setting, a fairly accurate diagnosis can be achieved. The role of magnetic resonance imaging (MRI) and radionuclide scanning is to differentiate soft-tissue infection from osteomyelitis. [8] An indium-111 WBC scan often is used because it is more specific than the technetium-99m scan.
Radiographic findings in the early stages of neuropathic arthropathy (Charcot joint) may simulate osteoarthritis. Radiographs may not demonstrate findings that help in diagnosing osteomyelitis in neuropathic joints, which is a common problem.
The roles of ultrasonography and CT scanning are limited. Ultrasonography can be used to identify any local collection when infection occurs and to guide aspiration for cytologic analysis; however, it provides no further information regarding the integrity of underlying bone. Although CT scanning may be helpful in evaluating cortical destruction, sequestra, and intraosseous gas, these changes are not specific for neuropathic arthropathy.
Bone marrow edema is nonspecific and has several causes; therefore, differentiating bone marrow edema from neuropathic arthropathy (Charcot joint) may not be possible on the basis of MRI findings alone. Similarly, enhanced bone activity on radionuclide scans is a nonspecific finding and may occur with several neoplastic, inflammatory, and degenerative processes.
Differential diagnosis and other problems to be considered
Calcium pyrophosphate deposition disease and primary osteoarthritis are in the differential diagnosis. In addition, in the early stage, neuropathic arthropathy (Charcot joint) can simulate osteoarthritis, and bone fragmentation and collapse are seen in osteonecrosis, posttraumatic osteoarthritis, intra-articular steroid injection, infection, and alkaptonuria. [9, 10, 11, 12]
Baker et al reviewed neuropathic arthropathy in diabetics. [13] Neuropathic diabetic arthropathy, particularly in the feet, is the leading cause of morbidity in diabetic patients. It has many mimics. Ulcers, sinus tracts, or an abscess with an adjacent region of abnormal signal intensity in bone marrow suggests osteomyelitis. Contrast-enhanced MRI allows differentiation of viable tissue from necrotic regions in diabetic foot infections, which require surgical debridement in addition to antibiotic therapy. Subtraction images are particularly useful for visualizing nonviable tissue.
Dialysis-associated spondyloarthropathy occurs in diabetic patients with a long history of hemodialysis. Intervertebral disk space narrowing without T2 signal hyperintensity, extensive endplate erosions without endplate remodelling, and facet joint involvement are suggestive of spondyloarthropathy instead of infectious discitis or degenerative disc disease. The clinical features of infective discitis and spondyloarthropathy overlap, but knowledge of the patient's medical history, as well as recognition of imaging characteristics described above, allows the radiologist to make a prompt and accurate diagnosis, leading to appropriate treatment. [13]
Guidelines
The National Institute for Health and Care Excellence (NICE) of the United Kingdom has published a guideline for the prevention and management of diabetic foot disorders. Recommendations specifically relevant to Charcot arthropathy in diabetes are as follows [14] :
-
If a person with diabetes fractures a foot or ankle, the fracture may progress to Charcot arthropathy.
-
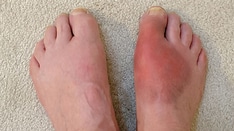
Acute Charcot arthropathy should be suspected if redness, warmth, swelling, or deformity (particularly if the skin is intact) is noted, especially in the presence of peripheral neuropathy or renal failure. Acute Charcot arthropathy should be considered even when deformity is not present or pain is not reported.
-
If acute Charcot arthropathy is suspected, a weightbearing radiograph of the affected foot and ankle should be obtained. Magnetic resonance imaging (MRI) may be considered if the radiograph is normal but Charcot arthropathy is still suspected.
The 2017 International Diabetic Foundation (IDF) guidelines on the diagnois and management of the diabetic foot also address Charcot arthropathy and include the following recommendations [1] :
-
In all patients presenting with a new diabetic foot infection (DFI), serial plain radiographs of the affected foot should be obtained to identify bone abnormalities (deformity, destruction) as well as soft-tissue gas and radiopaque foreign bodies.
-
Serial radiographs should be used to reassess potential osseous changes when healing progresses slowly or signs and/or symptoms worsen.
-
For patients who require additional imaging, particularly when soft-tissue abscess is suspected or the diagnosis of osteomyelitis remains uncertain, MRI is the study of choice.
-
In the acute phase, Charcot arthropathy may appear clinically similar to osteomyelitis. MRI findings consistent with an infection generally include soft-tissue fluid collection, sinus tracts, and diffuse marrow edema.
Radiography
Radiologic features of neuropathic arthropathy (Charcot joint) are the same irrespective of the etiology and distribution. Early stage radiographic findings include persistent or progressive joint effusion, narrowing of the joint space, soft-tissue calcification, minimal subluxation, preservation of bone density (unless infected), and fragmentation of eburnated subchondral bone. Neuropathy-like arthropathy can be seen in patients with calcium pyrophosphate dihydrate deposition disease.
Radiographic features found in the severe form of neuropathic arthropathy are pathognomonic, and no further imaging is necessary. Bone eburnation, fracture, subluxation, and joint disorganization can be more profound in this disorder. The bone collapse seen in the late stage may resemble osteonecrosis and posttraumatic osteoarthritis. Radiography is a relatively insensitive modality when used in the diagnosis of osteomyelitis. Bone fragmentation and collapse are manifestations of osteonecrosis, posttraumatic osteoarthritis, intra-articular steroid arthropathy, infection, and alkaptonuria. In the late stage, there is radiographic evidence of destruction of articular surfaces, subchondral sclerosis, osteophytosis, intra-articular loose bodies (bag of bones), subluxation, Lisfranc fracture/dislocation of midtarsal bones, and rapid bone resorption demonstrating pencil-in-a-cup deformity (see the images below). Complications of septic arthritis that are demonstrated on radiographs include osteomyelitis and bone ankylosis. [6]
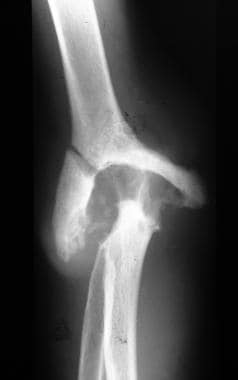 Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with syringomyelia. Anteroposterior view of the elbow demonstrates resorption of the bone with opaque subchondral bone.
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with syringomyelia. Anteroposterior view of the elbow demonstrates resorption of the bone with opaque subchondral bone.
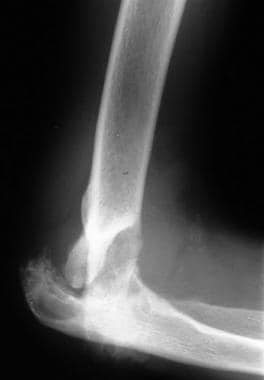 Neuropathic arthropathy (Charcot joint). Lateral view of the elbow shows disorganization of the joint (same patient as in the previous image).
Neuropathic arthropathy (Charcot joint). Lateral view of the elbow shows disorganization of the joint (same patient as in the previous image).
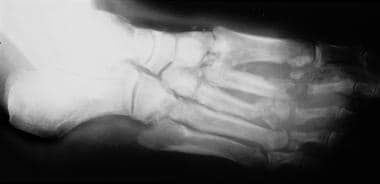 Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with diabetes mellitus. Note the lateral disruption of the base of the metatarsal in relation to the tarsals, representing a Lisfranc fracture/dislocation. Note the soft-tissue gas and osteomyelitis of the second and third metatarsal heads.
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with diabetes mellitus. Note the lateral disruption of the base of the metatarsal in relation to the tarsals, representing a Lisfranc fracture/dislocation. Note the soft-tissue gas and osteomyelitis of the second and third metatarsal heads.
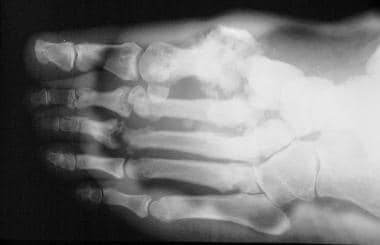 Neuropathic arthropathy (Charcot joint). Lisfranc fracture/dislocation in a patient with diabetes and neuropathic arthropathy. Note the soft-tissue swelling, fragmentation, sclerosis, and periostitis.
Neuropathic arthropathy (Charcot joint). Lisfranc fracture/dislocation in a patient with diabetes and neuropathic arthropathy. Note the soft-tissue swelling, fragmentation, sclerosis, and periostitis.
 Neuropathic arthropathy (Charcot joint). Osteolysis of the distal metatarsals and phalanges with tapering results in a pencil-like appearance in the late stage of diabetic neuropathy.
Neuropathic arthropathy (Charcot joint). Osteolysis of the distal metatarsals and phalanges with tapering results in a pencil-like appearance in the late stage of diabetic neuropathy.
Distribution of joint disease varies depending on the etiology. In diabetic neuropathy, common sites of involvement are the metatarsophalangeal, tarsometatarsal, and intertarsal joints (see the images below).
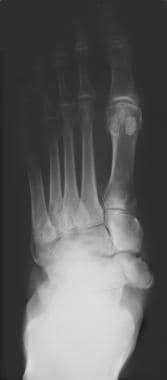 Neuropathic arthropathy (Charcot joint). Anteroposterior view of the foot in a patient with diabetes and neuropathic arthropathy. Note the fragmentation, collapse, and sclerosis of the intertarsal joints.
Neuropathic arthropathy (Charcot joint). Anteroposterior view of the foot in a patient with diabetes and neuropathic arthropathy. Note the fragmentation, collapse, and sclerosis of the intertarsal joints.
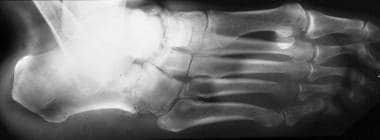 Neuropathic arthropathy (Charcot joint). Oblique view of the foot in a patient with diabetes and neuropathic arthropathy shows destruction of the articular surface of the intertarsal joints with subchondral sclerosis (same patient as in the previous image).
Neuropathic arthropathy (Charcot joint). Oblique view of the foot in a patient with diabetes and neuropathic arthropathy shows destruction of the articular surface of the intertarsal joints with subchondral sclerosis (same patient as in the previous image).
In syringomyelia, neuropathic changes are relatively more common in the shoulder joint, followed by the elbow [15] and wrist. The lower extremities can also be affected in syringomyelia. Changes in the spine are most characteristic in the cervical region.
 Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy of the shoulder in a patient with syringomyelia. Note the destruction of the articular surface, dislocation, and debris, which are pathognomonic for a neuropathic joint.
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy of the shoulder in a patient with syringomyelia. Note the destruction of the articular surface, dislocation, and debris, which are pathognomonic for a neuropathic joint.
 Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with syringomyelia. Anteroposterior view of the elbow demonstrates resorption of the bone with opaque subchondral bone.
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with syringomyelia. Anteroposterior view of the elbow demonstrates resorption of the bone with opaque subchondral bone.
 Neuropathic arthropathy (Charcot joint). Lateral view of the elbow shows disorganization of the joint (same patient as in the previous image).
Neuropathic arthropathy (Charcot joint). Lateral view of the elbow shows disorganization of the joint (same patient as in the previous image).
The joints of the lower extremity are commonly affected in patients with tabes dorsalis (see the images below). Other common sites include the joints of the forefoot and midfoot and the vertebral column.
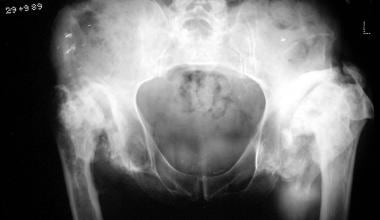 Neuropathic arthropathy (Charcot joint). Gross disorganization of the hip joints in a patient with tabes dorsalis.
Neuropathic arthropathy (Charcot joint). Gross disorganization of the hip joints in a patient with tabes dorsalis.
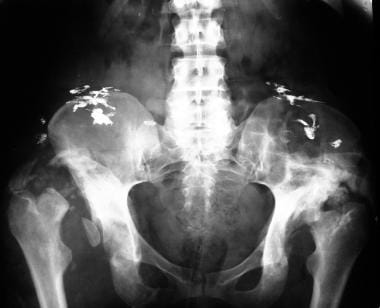 Neuropathic arthropathy (Charcot joint). Charcot joint in a patient with tabes dorsalis who has dislocation, osseous fragmentation, and sclerosis. The opacities projected over the iliac blades represent intramuscular bismuth-containing injections.
Neuropathic arthropathy (Charcot joint). Charcot joint in a patient with tabes dorsalis who has dislocation, osseous fragmentation, and sclerosis. The opacities projected over the iliac blades represent intramuscular bismuth-containing injections.
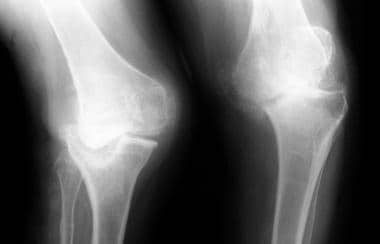 Neuropathic arthropathy (Charcot joint). Fragmentation and collapse of the chondral and osseous structures of both knee joints in a patient with tabes dorsalis.
Neuropathic arthropathy (Charcot joint). Fragmentation and collapse of the chondral and osseous structures of both knee joints in a patient with tabes dorsalis.
The ankle and intertarsal joints are commonly involved in patients with myelomeningocele and congenital insensitivity to pain (asymbolia). The interphalangeal joints of the hands and the metatarsophalangeal joints of the feet are commonly affected in patients with leprosy (see the following image). Neuropathic arthropathy associated with chronic alcoholism commonly involves the metatarsophalangeal and interphalangeal joints. The knee and ankle appear to be the predominant sites of involvement in patients with amyloidosis.
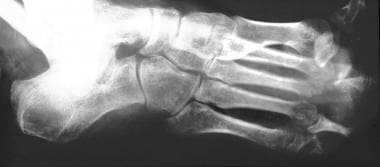 Neuropathic arthropathy (Charcot joint). Anteroposterior and oblique views of a neuropathic foot in a patient with leprosy. Note the predominant involvement of the metatarsophalangeal and interphalangeal joints with severe periostitis.
Neuropathic arthropathy (Charcot joint). Anteroposterior and oblique views of a neuropathic foot in a patient with leprosy. Note the predominant involvement of the metatarsophalangeal and interphalangeal joints with severe periostitis.
Computed Tomography
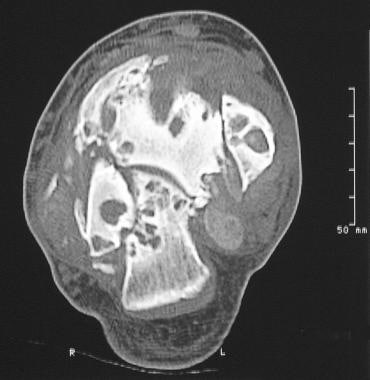
CT scanning has no significant role in the diagnosis of neuropathic arthropathy. However, CT scans may be helpful in evaluating cortical destruction, sequestra, and intraosseous gas (see the following image). [16]
Magnetic Resonance Imaging
On T1-weighted MRIs, joints involved in neuropathic arthropathy (Charcot joint) appear diffusely swollen and demonstrate low signal intensity. The fat plane adjacent to the skin ulceration appears hypointense; when the joints are infected with a gas-producing organism, areas showing a loss of signal intensity are seen. After the intravenous administration of a gadolinium-based contrast agent, the inflammatory mass enhances and demonstrates central nonenhancing necrotic debris.
MRI plays a significant role in diagnosing complications. Findings help in assessing the extent of the disease and in determining the presence of osteomyelitis. Differentiating bone changes that result from neuropathic arthropathy (Charcot joint) from those that result from infection is difficult. Bone marrow edema is a nonspecific finding that can be seen in both neuropathic arthropathy and in infection. In general, bone marrow edema found close to a skin ulceration and away from a joint suggests infection.
On short-tau inversion recovery (STIR) sequences, early bone infection may be evidenced by high-signal marrow edema. Later, loss of clarity of the cortical outline and cortical destruction can be identified.
Features that help differentiate spinal neuroarthropathy from disc infection include joint disorganization; facet involvement; debris; a pattern of diffuse signal intensity in the vertebral bodies; spondylolisthesis; and rim enhancement of the disc on gadolinium-enhanced MRIs. [17] Features that do not help in differentiation include endplate sclerosis, erosions, osteophytes, a reduction in disc height, and paraspinal soft-tissue masses.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). For more information, see the eMedicine topic Nephrogenic Systemic Fibrosis. The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness. For more information, see the FDA Public Health Advisory or Medscape.
Nuclear Imaging
The role of radioisotopic studies is to detect osteomyelitis in a neuropathic joint. [18] Three-phase phosphate scintigraphy has a high sensitivity (85%) but a low specificity (55%) because of bone remodeling of other causes. Studies using uptake of the gallium-67 (67Ga) citrate have a high false-positive rate. Scanning using indium-111 (111In)–labeled leukocytes has the highest sensitivity (87%) and specificity (81%) for detecting osteomyelitis in a neuropathic foot. The role of positron emission tomography (PET) scanning with fluorodeoxyglucose (FDG) is promising. [19]
One study has shown a valuable role of FDG-PET scanning in the setting of neuroarthropathic arthropathy (Charcot joint) by reliably differentiating it from osteomyelitis, both in general and when foot ulcer is present. [20] In diabetic patients in the setting of concomitant foot ulcer, FDG-PET scanning accurately ruled out osteomyelitis. Basu and associates estimated a 100% sensitivity and 93.8% accuracy of FDG-PET scanning in the diagnosis of Charcot foot; by contrast, MRI had a sensitivity of 76.9% and an accuracy of 75%. [20]
The incidence of false-positive results is increased because of coexisting bone remodeling.
-
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy of the shoulder in a patient with syringomyelia. Note the destruction of the articular surface, dislocation, and debris, which are pathognomonic for a neuropathic joint.
-
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with syringomyelia. Anteroposterior view of the elbow demonstrates resorption of the bone with opaque subchondral bone.
-
Neuropathic arthropathy (Charcot joint). Lateral view of the elbow shows disorganization of the joint (same patient as in the previous image).
-
Neuropathic arthropathy (Charcot joint). Gross disorganization of the hip joints in a patient with tabes dorsalis.
-
Neuropathic arthropathy (Charcot joint). Charcot joint in a patient with tabes dorsalis who has dislocation, osseous fragmentation, and sclerosis. The opacities projected over the iliac blades represent intramuscular bismuth-containing injections.
-
Neuropathic arthropathy (Charcot joint). Fragmentation and collapse of the chondral and osseous structures of both knee joints in a patient with tabes dorsalis.
-
Neuropathic arthropathy (Charcot joint). Anteroposterior view of the foot in a patient with diabetes and neuropathic arthropathy. Note the fragmentation, collapse, and sclerosis of the intertarsal joints.
-
Neuropathic arthropathy (Charcot joint). Oblique view of the foot in a patient with diabetes and neuropathic arthropathy shows destruction of the articular surface of the intertarsal joints with subchondral sclerosis (same patient as in the previous image).
-
Neuropathic arthropathy (Charcot joint). Computed tomography scan of the ankle in a patient with neuropathic arthropathy. Note the destruction of the articular surface, disorganization of the joint, and fragmentation.
-
Neuropathic arthropathy (Charcot joint). Anteroposterior and oblique views of a neuropathic foot in a patient with leprosy. Note the predominant involvement of the metatarsophalangeal and interphalangeal joints with severe periostitis.
-
Neuropathic arthropathy (Charcot joint). Neuropathic arthropathy in a patient with diabetes mellitus. Note the lateral disruption of the base of the metatarsal in relation to the tarsals, representing a Lisfranc fracture/dislocation. Note the soft-tissue gas and osteomyelitis of the second and third metatarsal heads.
-
Neuropathic arthropathy (Charcot joint). Lisfranc fracture/dislocation in a patient with diabetes and neuropathic arthropathy. Note the soft-tissue swelling, fragmentation, sclerosis, and periostitis.
-
Neuropathic arthropathy (Charcot joint). Osteolysis of the distal metatarsals and phalanges with tapering results in a pencil-like appearance in the late stage of diabetic neuropathy.