Practice Essentials
Optic neuritis (ON) is an inflammatory condition of the optic nerve that is commonly seen in central nervous system demyelinating diseases such as multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-related diseases. [1, 2, 3]
The diagnosis of optic neuritis (inflammation of the optic nerve) is usually made clinically, with direct imaging of the optic nerves by magnetic resonance imaging (MRI) being reserved for atypical cases. Optic neuritis, which is one of the causes of acute loss of vision associated with pain, can be the initial episode for a patient who will subsequently develop multiple sclerosis (MS). MRI of the brain provides information that can change the management of optic neuritis and yields prognostic information regarding the patient's future risk for the development of multiple sclerosis. [4, 5, 6, 7, 8] As established by the Optic Neuritis Treatment Trial, an abnormal baseline brain MRI scan is a strong predictor of MS after isolated optic neuritis in adults. [4, 9, 10, 11, 1] In atypical cases (eg, prolonged or severe pain, lack of visual recovery, atypical visual-field loss, evidence of orbital inflammation and/or inflammation), MRI is used to further characterize and to exclude other disease processes. [2]
(See the images below.)
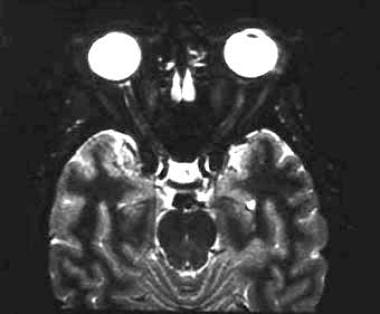 A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, short tau inversion recovery (STIR) image demonstrates faint increased signal in the distal left optic nerve.
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, short tau inversion recovery (STIR) image demonstrates faint increased signal in the distal left optic nerve.
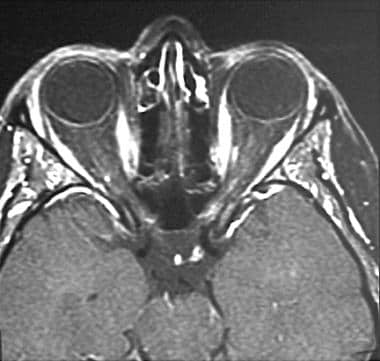 A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fat-suppressed, postcontrast, T1-weighted image demonstrates enhancement in the intracanalicular portion of the left optic nerve.
A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fat-suppressed, postcontrast, T1-weighted image demonstrates enhancement in the intracanalicular portion of the left optic nerve.
Computed tomography (CT) scanning has a very limited role in the setting of optic neuritis. Size differences in the optic nerve can be appreciated, but this is neither sensitive nor specific. Contrast-enhanced CT scanning of the orbits may be able to help exclude other orbital pathology, albeit in a limited way relative to MRI, because of the inherently superior soft-tissue contrast resolution yielded with MRI. Certainly, CT scanning of the brain, regardless of whether intravenous contrast material is administered or not, does not yield prognostic and treatment-altering information as does MRI of the brain. [12]
Studies of optical coherence tomography (OCT) have shown that it may be of value in monitoring acute optic neuritis. [12, 13]
Magnetic Resonance Imaging
Thin (2-3mm), fat-suppressed, T2-weighted images, such as short tau inversion recovery (STIR) sequences, through the optic nerves may show characteristic high-signal intensity foci in the minimally expanded or nonexpanded nerve. These lesions frequently enhance following intravenous contrast administration, which is not seen in a healthy optic nerve. Some studies have shown that certain findings, such as optic nerve lesions of greater length and in certain locations (within the optic canal), may be associated with a worse visual prognosis and may benefit from certain treatments, but other studies have not supported this conclusion.
The signal intensity ratio (SIR) of the optic nerve to the white matter (WM) on STIR was found to be of diagnostic value for acute optic neuritis (AON) in a study of 405 patients with suspected orbital diseases who underwent orbital MRI with a 3-T scanner. SIRave and SIRmax were significantly (P< 0.001) higher in patients with acute optic neuritis than in control patients. At a cut-off SIRave value of 1.119, the sensitivity, specificity, and accuracy were 0.939, 0.840, and 0.870, respectively. At a cut-off SIRmax value of 1.281, sensitivity, specificity, and accuracy were 1.000, 0.720 and 0.806, respectively. [14]
Optic neuritis in neuromyelitis optica was found to have a distinct pattern on MRI, as compared to relapsing-remitting multiple sclerosis. The majority of neuromyelitis optica lesions were longitudinally extensive, measuring at least 17.6 mm in length and involving at least 3 optic nerve segments. At a cutoff of 17.6 mm lesion length, the specificity for neuromyelitis optica was 76.9%; sensitivity, 80.8%; and positive likelihood, 3.50. Conversely, multiple sclerosis lesions were more commonly focal in one optic nerve segment localized anteriorly. [5]
In 25 patients with optic neuritis, multi-scale MRI texture analysis was used to assess optic nerve pathology. Retinal nerve fiber layer (RNFL) values were 20% thicker and lesion texture was 14% more heterogeneous in affected eyes than in nonaffected eyes. In addition, the lesion texture ratio of affected to nonaffected eyes was greater in patients than in controls. In the affected eyes, visual acuity recovered significantly over 6 months (18/23 patients) and 12 months (18/21 patients) when RNFL thickness and optic nerve area ratio decreased over time. Texture heterogeneity in the standard MRI of acute optic nerve lesions was the only measure that predicted functional recovery after optic neuritis. The authors concluded that tissue heterogeneity may be a potential measure of functional outcome in optic neuritis patients and that advanced analysis of the texture in standard MRI could provide insights into mechanisms of injury and recovery in patients with similar disorders. [6]
Diffusion-weighted and diffusion-tensor imaging may contribute more data that may prove to have some bearing on treatment and/or on prognosis. [15, 16, 17, 18] The thought is that the loss of anisotropy (manifested by an increase in the apparent diffusion coefficient or a decrease in fractional anisotropy) associated with demyelination and/or axonal damage may be more sensitive and/or yield more prognostic information than anatomic imaging findings (size, T2 signal intensity, and enhancement, which suggests loss of the blood-brain barrier due to the underlying pathologic process), which could manifest themselves much later than the findings associated with loss of anisotropy. However, with the current technology, diffusion-weighted and diffusion-tensor imaging of the optic nerves is too time- and labor-intensive for broad clinical application.
In a study of 31 patients with optic neuritis, 3-dimensional double inversion recovery (3D DIR) was found to provide improved detection of optic nerve signal abnormalities over 2-dimensional (2D) short tau inversion recovery (STIR) fluid-attenuated inversion recovery (FLAIR). Multiplanar DIR images had the best performance for the diagnosis of optic neuritis, with a sensitivity of 95% and a specificity of 94%. [19]
In a study of 42 patients with suspected optic neuritis, perioptic leptomeningeal contrast enhancement on fat-suppressed FLAIR images was identified as a novel marker in optic neuritis, possibly reflecting a leptomeningeal inflammatory process preceding or accompanying optic neuritis. The authors suggested that thin-section contrast-enhanced fat-suppressed FLAIR images may be a useful addition in MRI protocols for patients with suspected optic neuritis. [20]
(MRI characteristics of optic neuropathy are demonstrated in the images below.)
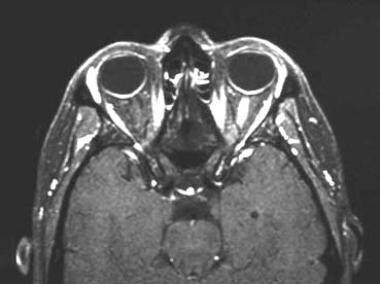 A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, fat-suppressed, postgadolinium, T1-weighted image through the orbit reveals an intensely enhancing segment of the distal left optic nerve.
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, fat-suppressed, postgadolinium, T1-weighted image through the orbit reveals an intensely enhancing segment of the distal left optic nerve.
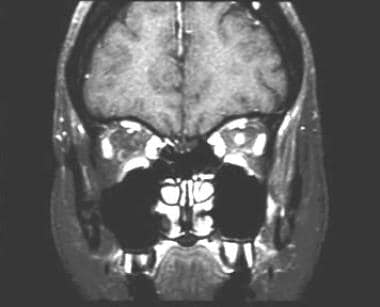 A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Coronal, fat-suppressed, postgadolinium, T1-weighted image demonstrates intense enhancement within the optic nerve. No significant nerve expansion or enhancement of the adjacent tissues is seen. Note the normal right optic nerve for comparison.
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Coronal, fat-suppressed, postgadolinium, T1-weighted image demonstrates intense enhancement within the optic nerve. No significant nerve expansion or enhancement of the adjacent tissues is seen. Note the normal right optic nerve for comparison.
 A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fat-suppressed, postcontrast, T1-weighted image demonstrates enhancement in the intracanalicular portion of the left optic nerve.
A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fat-suppressed, postcontrast, T1-weighted image demonstrates enhancement in the intracanalicular portion of the left optic nerve.
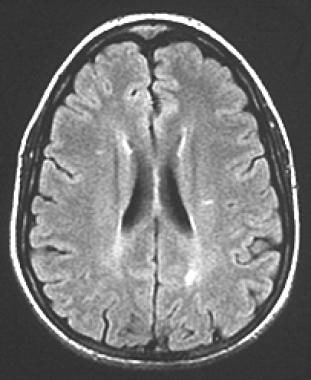 A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fluid-attenuated inversion recovery (FLAIR) image demonstrates bilateral periventricular white matter lesions. Several additional and similar lesions were seen in other locations (not shown). No history of prior neurologic illness was noted in the patient, but in the setting of acute optic neuritis, the multiple white matter lesions in a number and pattern atypical for the patient's age were considered to be supportive of the diagnosis of multiple sclerosis.
A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fluid-attenuated inversion recovery (FLAIR) image demonstrates bilateral periventricular white matter lesions. Several additional and similar lesions were seen in other locations (not shown). No history of prior neurologic illness was noted in the patient, but in the setting of acute optic neuritis, the multiple white matter lesions in a number and pattern atypical for the patient's age were considered to be supportive of the diagnosis of multiple sclerosis.
Optic neuritis and MS
The real contribution of imaging in the setting of optic neuritis is made by imaging of the brain, not of the optic nerves themselves. This is due to the fact that the most valuable predictor for the development of subsequent MS is the presence of white matter abnormalities. Between 27% and 70% of patients (in various studies) with isolated optic neuritis show abnormal MRI brain findings, as defined by the presence of 2 or more white matter lesions on T2-weighted images. [4, 9, 7, 21, 22, 23]
In the Optic Neuritis Treatment Trial, the 5-year risk of developing MS was 16% in patients with normal brain MRI findings, 37% with 1-2 lesions, and 51% with 3 or more lesions. At 10 years, the only statistically significant difference was between no lesions (22% risk) and 1 or more lesions (56% risk). [24]
Bonhomme et al found that children with brain MRI abnormalities at the time of optic neuritis diagnosis had an increased risk for MS. The investigators studied the rate of conversion to MS after a diagnosis of optic neuritis in children (younger than 18 yr) who presented with optic neuritis between at the Children's Hospital of Philadelphia. [4] In the study, Bonhomme and colleagues identified 29 children with idiopathic optic neuritis. Eleven of the 29 patients (38%) had white matter T2/FLAIR (fluid-attenuated inversion recovery) lesions in the brain (not including the optic nerves). Eighteen patients were followed for more than 24 months, and 3 of the 18 (17%) developed MS. All 3 patients who developed MS had an abnormal brain MRI scan at their initial presentation of optic neuritis. None of the patients who had normal MRI scans developed MS over an average follow-up period of 88.5 months. [4]
Swanton et al found that the presence and number of spinal cord lesions at baseline and of new T2 lesions at follow-up were significant independent predictors of higher disability. In their report, the investigators studied patients with optic neuritis to determine the influence of lesion number, location, and activity, as well as non-lesion MRI measures obtained on early scans. At 6-year follow-up, 48% of patients had converted to clinically definite MS, and 52% remained with clinically isolated syndrome. [7]
Disability was also predicted by the presence at baseline of gadolinium-enhancing lesions and the number of infratentorial lesions. Only spinal cord lesions predicted disability in patients converting to clinically definite MS.
The Optic Neuritis Treatment Trial found that there was a 50% cumulative probability of developing MS within 15 years after the onset of optic neuritis. The presence of lesions on a baseline, non–contrast-enhanced MRI of the brain was a significant factor in the occurrence of MS. The study also found that in patients with optic neuritis who had no lesions on baseline brain MRI, 25% developed MS during follow-up, while among patients with 1 or more lesions, 72% developed MS. [9]
In a study of 34 children with isolated optic neuritis, abnormal cranial MRI (cMRI) was found to be associated with an increase in the odds of MS development (odds ratio 20.57; 95% CI 2.16-196.10, P< 0.001). Positive oligoclonal bands in spinal fluid was also found to be associated with MS, but only cMRI remained statistically significant in multiple regressions. [21]
Influence of MRI on treatment
Information from brain MRI has a potential influence on treatment. It has been shown that in 2-year follow-up of patients with optic neuritis and 2 or more brain lesions on MRI scans, patients given intravenous methylprednisolone (as compared with placebo and oral prednisone groups) had a significantly decreased risk of developing MS. Note that this benefit was not maintained at 3 years. [25, 26]
In a study using interferon beta-1a in patients with optic neuritis with 2 or more white matter lesions on MRI scans of the brain, a decreased risk of developing MS at 3 years was demonstrated. In those patients who did ultimately develop MS, interferon beta-1a was shown to reduce the disease burden and number of active lesions. [27, 28]
False positives/negatives
Although not specifically relevant to optic neuritis, false-positive results in orbital imaging can result from failure of complete fat saturation related to magnetic susceptibility artifact from dental amalgam and air–soft tissue interfaces, particularly at the inferior margin of the orbit. This is true especially for frequency-selective fat-saturation techniques but less so for inversion recovery sequences.
Fat-saturation failure can mimic orbital edema on multiecho-train, T2-weighted images or enhancement on fat-suppressed, T1-weighted images. Careful evaluation of the tissue surrounding the orbit should reveal the true cause of signal distortion. This artifact should not occur in optic neuritis, because the lesion of optic neuritis is confined to the nerve, but it can potentially mislead the interpreter to conclude that more diffuse orbital inflammation is the cause of vision loss.
Occasionally, the enhancement pattern in optic neuritis is a peripheral tram-track pattern. Potentially, this can be confused with the enhancement pattern of optic nerve meningioma. However, the optic neuritis pattern should be distinguished from the meningioma pattern by enhancement limited to the nerve, rather than the sheathlike pattern of meningioma; by the absence of significant mass or expansion; and by the clinical features of acute-onset vision loss and pain.
Questions & Answers
Overview
How is optic neuritis diagnosed?
Which imaging modalities are used in the diagnosis of optic neuritis?
What is the role of MRI in the diagnosis of optic neuritis?
Which findings on MRI are characteristic of optic neuritis?
What is the role of MRI in the diagnosis of optic neuritis in multiple sclerosis (MS)?
How do brain MRI findings of optic neuritis affect the treatment of multiple sclerosis (MS)?
What is the accuracy of MRI for optic neuritis imaging?
-
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, short tau inversion recovery (STIR) image demonstrates faint increased signal in the distal left optic nerve.
-
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Axial, fat-suppressed, postgadolinium, T1-weighted image through the orbit reveals an intensely enhancing segment of the distal left optic nerve.
-
A 43-year-old woman with acute vision loss and eye pain. No prior neurologic symptoms were noted. Coronal, fat-suppressed, postgadolinium, T1-weighted image demonstrates intense enhancement within the optic nerve. No significant nerve expansion or enhancement of the adjacent tissues is seen. Note the normal right optic nerve for comparison.
-
A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fat-suppressed, postcontrast, T1-weighted image demonstrates enhancement in the intracanalicular portion of the left optic nerve.
-
A 35-year-old woman with acute onset of left-eye pain and vision decline. Axial, fluid-attenuated inversion recovery (FLAIR) image demonstrates bilateral periventricular white matter lesions. Several additional and similar lesions were seen in other locations (not shown). No history of prior neurologic illness was noted in the patient, but in the setting of acute optic neuritis, the multiple white matter lesions in a number and pattern atypical for the patient's age were considered to be supportive of the diagnosis of multiple sclerosis.










