Practice Essentials
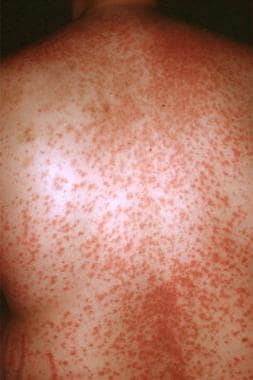
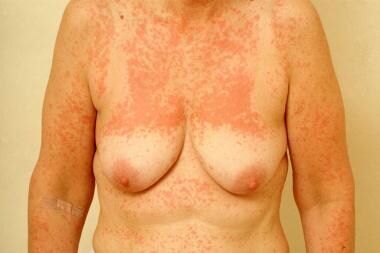
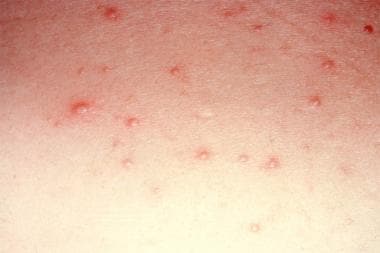
Drug eruptions can mimic a wide range of dermatoses. They may be related to traditional pharmaceutical agents, biologic agents and targeted immunotherapeutic agents, food additives, and dyes. [1, 2, 3, 4, 5] The morphologies are myriad and include morbilliform (see the image below), urticarial, papulosquamous, pustular, and bullous.
Medications can also cause pruritus and dysesthesia without an obvious eruption. A drug-induced reaction should be considered in any patient who is taking medications and who suddenly develops a symmetric cutaneous eruption. Medications that are known for causing cutaneous reactions include antimicrobial agents, [6] nonsteroidal anti-inflammatory drugs (NSAIDs), cytokines, chemotherapeutic agents, anticonvulsants, and psychotropic agents. Morbilliform eruption localized to striae has been described with clindamycin. [7]
Signs and symptoms
The first steps in the history are as follows:
-
Review the patient’s complete medication list, including prescription and over-the-counter (OTC) drugs
-
Document any history of previous adverse reactions to drugs or foods
-
Consider alternative etiologies (eg, viral exanthems and bacterial infections)
-
Note any concurrent infections, metabolic disorders, or immunocompromise
In addition, the following should be noted and detailed:
-
Interval between introduction of a drug and onset of the eruption
-
Route, dose, duration, and frequency of drug administration
-
Use of parenterally administered drugs (more likely to cause anaphylaxis)
-
Use of topically applied drugs (more likely to induce delayed-type hypersensitivity)
-
Use of multiple courses of therapy and prolonged administration (risk of allergic sensitization)
-
Any improvement after drug withdrawal and any reaction with readministration
Physical examination should address clinical features that may indicate a severe, potentially life-threatening drug reaction, including the following:
-
Mucous membrane erosions
-
Blisters
-
Nikolsky sign
-
Confluent erythema
-
Angioedema and tongue swelling
-
Palpable purpura
-
Skin necrosis
-
Lymphadenopathy
-
High fever, dyspnea, or hypotension
It is important to appreciate the morphology and physical features of drug eruptions, as follows:
-
Acneiform
-
Acral erythema (erythrodysesthesia)
-
Acute generalized exanthematous pustulosis (AGEP)
-
Dermatomyositislike
-
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome
-
Erythema multiforme (EM), including EM minor, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap
-
Hypersensitivity syndrome
-
Lupus
-
Morbilliform or exanthematous
-
Sweet syndrome (acute febrile neutrophilic dermatosis)
-
Urticaria
-
Vesiculobullous
See Clinical Presentation for more detail.
Diagnosis
With mild asymptomatic eruptions, the history and physical examination are often sufficient for diagnosis; with severe or persistent eruptions, further diagnostic testing may be required, as follows:
-
Biopsy
-
Complete blood count (CBC) with differential
-
Serum chemistry studies (especially for electrolyte balance and indices of renal or hepatic function in patients with severe reactions)
-
Antibody or immunoserology tests
-
Direct cultures to investigate a primary infectious etiology or secondary infection
-
Urinalysis, stool guaiac tests, and chest radiography for vasculitis
-
Skin prick or patch testing to confirm the causative agent
See Workup for more detail.
Management
Principles of medical care are as follows:
-
The ultimate goal is to identify and discontinue the offending medication if possible
-
Patients can sometimes continue to be treated through morbilliform eruptions; nevertheless, all patients with severe morbilliform eruptions should be monitored for mucous membrane lesions, blistering, and skin sloughing
-
Treatment of a drug eruption depends on the specific type of reaction
-
Therapy for exanthematous drug eruptions is supportive, involving the administration of oral antihistamines, topical steroids, and moisturizing lotions
-
Severe reactions (eg, SJS, TEN, and hypersensitivity reactions) warrant hospital admission
-
Hypersensitivity syndrome may have to be treated with liver transplantation if the offending drug is not stopped in time; treatment with systemic corticosteroids has been advocated in the acute phase; in the chronic phase, patients may require treatment for hypothyroidism or diabetes mellitus
Prompt identification and withdrawal of the offending agent may help limit the toxic effects associated with the drug. The decision to discontinue a potentially vital drug often presents a dilemma. For most drug eruptions, full recovery without any complications is expected; however, the following should be noted:
-
Patients with exanthematous eruptions should expect mild desquamation as the rash resolves
-
Patients with hypersensitivity syndrome are at risk of becoming hypothyroid, usually within the first 4-12 weeks after the reaction; there is also a risk of diabetes
-
The prognosis for patients with TEN is guarded; scarring, blindness, and death are possible
Oncodermatology
Oncodermatology is increasing in importance as more patients are being treated with chemotherapeutic agents that modulate immune response and are associated with expected cutaneous reactions. Often, clinicians are called upon to manage drug eruptions in this setting while affected individuals are kept on the triggering therapy because of life saving or life sustaining benefits. Unusual reactions such as vitiligo may result from some medications such as cyclin dependent kinase inhibitors. [12]
Vaccinations
DRESS syndrome has been reported after vaccination for SARS COVID-19. [13] Vaccinations may contribute to other types of reactions as well.
See Treatment and Medication for more detail.
Pathophysiology
Drug eruptions may be divided into immunologically and nonimmunologically mediated reactions.
Immunologically mediated reactions
Coombs and Gell proposed four types of immunologically mediated reactions, as follows:
-
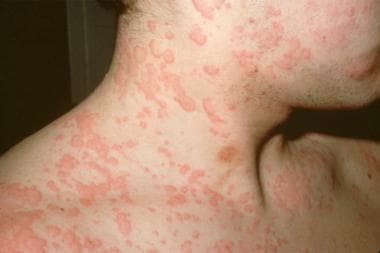
Type I - Immunoglobulin E (IgE)-dependent reactions, which result in urticaria, angioedema, and anaphylaxis (see the first image below)
-
Type II - Cytotoxic reactions, which result in hemolysis and purpura (see the second image below)
-
Type III - Immune complex reactions, which result in vasculitis, serum sickness, and urticaria
-
Type IV - Delayed-type reactions with cell-mediated hypersensitivity, which result in contact dermatitis, exanthematous reactions, and photoallergic reactions
Th17 T cells are implicated in many drug eruptions, and sulfamethoxazole induces a T-cell switch mechanism based on the TCRVβ20-1 domain altering peptide-HLA recognition. In severe drug reactions, micro RNA-18a-5p down-regulates the expression of the antiapoptotic B-cell lymphoma/leukemia-2–like protein 10 (BCL2L10), promoting apoptosis.
Insulin and other proteins are associated with type I reactions. Penicillin, cephalosporins, sulfonamides, and rifampin are known to cause type II reactions. Quinine, salicylates, chlorpromazine, and sulfonamides can cause type III reactions. Type IV reactions, the most common mechanism of drug eruptions, are often encountered in cases of contact hypersensitivity to topical medications (eg, neomycin). Sulfonamides are most frequently associated with TEN.
Although most drug eruptions are type IV hypersensitivity reactions, only a minority are IgE-dependent. That is, antibodies can be demonstrated in fewer than 5% of cutaneous drug reactions. Type IV cell-mediated reactions are not dose-dependent; they usually begin 7-20 days after the medication is started, they may involve blood or tissue eosinophilia, and they may recur if drugs chemically related to the causative agent are administered.
Nonimmunologically mediated reactions
Nonimmunologically mediated reactions may be classified according to the following features:
-
Accumulation
-
Adverse effects
-
Direct release of mast cell mediators
-
Idiosyncratic reactions
-
Intolerance
-
Jarisch-Herxheimer phenomenon
-
Overdosage
-
Phototoxic dermatitis
An example of accumulation is argyria (blue-gray discoloration of skin and nails) observed with use of silver nitrate nasal sprays.
Adverse effects are normal but unwanted effects of a drug. For example, antimetabolite chemotherapeutic agents, such as cyclophosphamide, are associated with hair loss.
Direct release of mast cell mediators is a dose-dependent phenomenon that does not involve antibodies. For example, aspirin and other NSAIDs cause a shift in leukotriene production, which triggers the release of histamine and other mast-cell mediators. Radiographic contrast material, alcohol, cytokines, opiates, cimetidine, quinine, hydralazine, atropine, vancomycin, and tubocurarine also may cause release of mast-cell mediators.
Idiosyncratic reactions are unpredictable and not explained by the pharmacologic properties of the drug. An example is the individual with infectious mononucleosis who develops a rash when given ampicillin.
Imbalance of endogenous flora may occur when antimicrobial agents preferentially suppress the growth of one species of microbe, allowing other species to grow vigorously. For example, candidiasis frequently occurs with antibiotic therapy.
Intolerance may occur in patients with altered metabolism. For example, individuals who are slow acetylators of the enzyme N-acetyltransferase are more likely than others to develop drug-induced lupus in response to procainamide.
Jarisch-Herxheimer phenomenon is a reaction due to bacterial endotoxins and microbial antigens that are liberated by the destruction of microorganisms. The reaction is characterized by fever, tender lymphadenopathy, arthralgias, transient macular or urticarial eruptions, and exacerbation of preexisting cutaneous lesions. This reaction can be seen with penicillin therapy for syphilis, griseofulvin or ketoconazole therapy for dermatophyte infections, and diethylcarbamazine therapy for oncocerciasis. It does not constitute an indication for stopping treatment; symptoms of Jarisch-Herxheimer reactions resolve with continued drug therapy, which should be maintained until the infection is fully eradicated.
Overdosage is an exaggerated response to an increased amount of a medication. For example, increased doses of anticoagulants may result in purpura.
Phototoxic dermatitis is an exaggerated sunburn response caused by the formation of toxic photoproducts, such as free radicals or reactive oxygen species (see the image below).
Etiology
Fibrosing reactions have been associated with a variety of chemical exposures. Nephrogenic systemic fibrosis has been associated with gadolinium contrast agents used in magnetic resonance imaging (MRI). Individuals with renal failure may have a buildup of gadolinium in the skin and other organs and may recruit CD34-positive bone marrow–derived fibrocytes into lesional areas. Toxic oil ingestion has been associated with morphea, and Texier disease has been associated with phytomenadione (vitamin K1) injections.
Rates of reactions to commonly used drugs are as follows:
-
Amoxicillin - 5.1%
-
Trimethoprim-sulfamethoxazole (TMP-SMX) - 4.7%
-
Ampicillin - 4.2%
-
Semisynthetic penicillin - 2.9%
-
Blood (whole human) - 2.8%
-
Penicillin G - 1.6%
-
Cephalosporins - 1.3%
-
Quinidine - 1.2%
-
Gentamicin sulfate - 1%
-
Packed red blood cells (RBCs) - 0.8%
-
Mercurial diuretics - 0.9%
-
Heparin - 0.7%
Cutaneous reaction rates in patients with HIV infection are as follows [14] :
-
Sulfasalazine - 20%
-
TMP-SMX - 14.9%
-
Aminopenicillins - 9.3%
-
Penicillins - 3.8%
-
Anticonvulsants - 3.4%
-
Dapsone - 3.1%
-
Penicillinase-resistant penicillins - 2.9%
-
Cephalosporins - 2.7%
-
Quinolones - 2.1%
-
Ketoconazole - 2%
-
Clindamycin - 1.8%
-
Primaquine - 1.8%
-
Tetracycline - 1.2%
-
Pentamidine - 1%
-
NSAIDs - 0.9%
-
Erythromycin - 0.6%
-
Zidovudine - 0.3%
Drugs that commonly cause serious reactions include the following:
-
Allopurinol
-
Anticonvulsants
-
Bumetanide
-
Captopril
-
Furosemide
-
NSAIDs
-
Penicillamine
-
Piroxicam
-
Sulfa drugs
-
Thiazide diuretics
Drugs unlikely to cause skin reactions include the following:
-
Acetaminophen
-
Aminophylline
-
Aspirin
-
Chlorpromazine
-
Codeine
-
Digoxin
-
Diphenhydramine hydrochloride
-
Ferrous sulfate
-
Folic acid
-
Meperidine
-
Methyldopa
-
Morphine
-
Prednisone
-
Prochlorperazine
-
Regular insulin
-
Serotonin-specific reuptake inhibitors
-
Tetracycline
-
Topical gels (eg, 4% tetracaine gel may cause serious cutaneous adverse reactions) [15]
-
Warfarin
Medications associated with specific types of cutaneous reactions
The following is a list of medications that have been reported to cause specific types of cutaneous reactions. However, not every possible type of drug eruption has been listed. In addition, exclusion of a drug from the following list does not imply that it is not the cause of a patient's eruption. A high index of suspicion must always be maintained when one is confronted with a new-onset eruption in a patient on multiple medications.
Acneiform
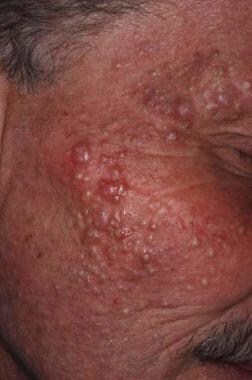
Causative agents include amoxapine, corticosteroids (see the image below), halogens, haloperidol, hormones, isoniazid, lithium, phenytoin, and trazodone.
Acute generalized exanthematous pustulosis
The most common causative agents include beta-lactam antibiotics and antibacterial sulfonamides, [16] diltiazem, antimalarials, macrolides, mercury, quinolones, and terbinafine.
Less common causative agents include acetaminophen, allopurinol, bufexamac, buphenine, carbamazepine, carbutamide, celecoxib, chloramphenicol, clindamycin, TMP-SMX, clobazam, cyclins (eg, tetracycline), cytarabine, famotidine, furosemide, ginkgo biloba, hydrochlorothiazide, hydroxychloroquine, ibuprofen, imatinib, imipenem, isoniazid, intravenous (IV) contrast dye, lopinavir-ritonavir, mexiletine, morphine, nadoxolol, nifedipine, nystatin, olanzapine, phenytoin, pipemidic acid, piperazine, pseudoephedrine, pyrimethamine, quinidine, ranitidine, rifampicin, salbutamine, sertraline, simvastatin, streptomycin, terbinafine, thallium, vancomycin, calcium-channel blockers, angiotensin-converting enzyme (ACE) inhibitors (eg, captopril, ramipril), glyburide, and gemfibrozil. [17, 18]
Alopecia
Causative agents include ACE inhibitors, allopurinol, anticoagulants, azathioprine, bromocriptine, beta blockers, cyclophosphamide, didanosine, hormones, indinavir, NSAIDs, phenytoin, methotrexate (MTX), retinoids, and valproate.
Bullous pemphigoid
Causative agents include aspirin, ampicillin, D-penicillamine, captopril, chloroquine, ciprofloxacin, enalapril, etanercept, [19] everolimus, furosemide, ibuprofen, levofloxacin neuroleptics, penicillins, phenacetin, psoralen plus UV-A, salicylazosulfapyridine, sirolimus, sulfasalazine, and terbinafine. [20]
Dermatomyositislike [21]
Causative agents include bacille Calmette-Guérin (BCG) vaccine, hydroxyurea (most common [22] ), lovastatin, omeprazole, penicillamine, simvastatin, and tegafur.
DRESS syndrome
The most common causative agents include aromatic anticonvulsants (phenytoin, phenobarbital [phenobarbitone], carbamazepine, lamotrigine, leviteracetam), sulfonamides, and antimicrobials (dapsone, TMP-SMX, piperacillin-tazobactam, vancomycin, abacavir, nevirapine, minocycline, and doxycycline). [23, 24]
Erythema nodosum
Causative agents include echinacea, halogens, oral contraceptives (most common), penicillin, sulfonamides, and tetracycline.
Erythroderma
Causative agents include allopurinol, anticonvulsants, aspirin, barbiturates, captopril, carbamazepine, cefoxitin, chloroquine, chlorpromazine, cimetidine, diltiazem, griseofulvin, lithium, nitrofurantoin, omeprazole, phenytoin, St John's wort, sulfonamides, and thalidomide.
Fixed drug eruptions
Causative agents include acetaminophen, ampicillin, anticonvulsants, aspirin/NSAIDs, barbiturates, benzodiazepines, butalbital, cetirizine, ciprofloxacin, clarithromycin, dapsone, dextromethorphan, doxycycline, fluconazole, hydroxyzine, lamotrigine, loratadine, metronidazole, oral contraceptives, penicillins, phenacetin, phenolphthalein, phenytoin, piperacillin-tazobactam, [25] piroxicam, saquinavir, sulfonamides, tetracyclines, ticlopidine, tolmetin, vancomycin, and zolmitriptan.
Hypersensitivity syndrome
Causative agents include allopurinol, amitriptyline, carbamazepine, dapsone, lamotrigine, minocycline, NSAIDs, olanzapine, oxcarbazepine, phenobarbital, phenytoin, saquinavir, spironolactone, sulfonamides, zalcitabine, and zidovudine.
Lichenoid
Causative agents include amlodipine, antimalarials, beta blockers, captopril, diflunisal, diltiazem, enalapril, furosemide, glimepiride, gold, leflunomide, levamisole, L-thyroxine, orlistat, penicillamine, phenothiazine, pravastatin, proton pump inhibitors (PPIs), rofecoxib, salsalate, sildenafil, tetracycline, thiazides, and ursodeoxycholic acid. [26]
Linear IgA dermatosis
Causative agents include atorvastatin, captopril, carbamazepine, diclofenac, glibenclamide, lithium, phenytoin, and vancomycin. [27]
Lupus erythematosus
Drug-induced systemic lupus erythematosus (SLE) is most commonly associated with hydralazine, procainamide, and minocycline. [28] Beta blockers, chlorpromazine, cimetidine, clonidine, estrogens, isoniazid, lithium, lovastatin, methyldopa, oral contraceptives, quinidine, sulfonamides, tetracyclines, and tumor necrosis factor (TNF)-α inhibitors have been reported. Drug-induced subacute cutaneous lupus erythematosus (SCLE) is most commonly associated with hydrochlorothiazide. Calcium-channel blockers, cimetidine, griseofulvin, leflunomide, terbinafine, and TNF-α inhibitors have been reported.
Morbilliform (exanthematous)
Causative agents include ACE inhibitors, allopurinol, amoxicillin, ampicillin, anticonvulsants, barbiturates, carbamazepine, cetirizine, ginkgo biloba, hydroxyzine, isoniazid, nelfinavir, NSAIDs, phenothiazine, phenytoin, quinolones, sulfonamides, thalidomide, thiazides, TMP-SMX, and zalcitabine.
Pemphigus
Causative agents include thiols and nonthiols. [29] Thiols include captopril, D-penicillamine, gold sodium thiomalate, mercaptopropionylglycine, pyritinol, thiamazole, and thiopronine. Nonthiols include aminophenazone, aminopyrine, azapropazone, cephalosporins, heroin, hydantoin, imiquimod, indapamide, levodopa, lysine acetylsalicylate, montelukast, oxyphenbutazone, penicillins, phenobarbital, phenylbutazone, piroxicam, progesterone, propranolol, and rifampin.
Photosensitivity
Causative agents include ACE inhibitors, amiodarone, amlodipine, celecoxib, chlorpromazine, diltiazem, furosemide, griseofulvin, lovastatin, nifedipine, phenothiazine, piroxicam, quinolones, sulfonamides, tetracycline, and thiazide.
Pseudoporphyria
Causative agents include amiodarone, bumetanide, chlorthalidone, cyclosporine, dapsone, etretinate, 5-fluorouracil (FU), flutamide, furosemide, hydrochlorothiazide/triamterene, isotretinoin, NSAIDs (including nalidixic acid and naproxen), oral contraceptive pills, and tetracycline
Psoriasis
Causative agents include ACE inhibitors, angiotensin receptor antagonists, antimalarials, beta-blockers, bupropion, calcium channel blockers, carbamazepine, interferon (IFN) alfa, lithium, metformin, NSAIDs, terbinafine, tetracyclines, valproate sodium, and venlafaxine. [30, 31, 32]
Serum sickness
Causative agents include antithymocyte globulin for bone marrow failure, human rabies vaccine, penicillin, pneumococcal vaccine (in AIDS patients), and vaccines containing horse serum derivatives. [33]
Serum sickness–like
Causative agents include beta-lactam antibiotics, [34] cefaclor (most common), minocycline, propranolol, streptokinase, sulfonamides, and NSAIDs.
Stevens-Johnson syndrome
Causative agents include allopurinol, anticonvulsants, aspirin/NSAIDS, barbiturates, carbamazepine, cimetidine, ciprofloxacin, codeine, didanosine, diltiazem, erythromycin, furosemide, griseofulvin, hydantoin, indinavir, nitrogen mustard, penicillin, phenothiazine, phenylbutazone, phenytoin, ramipril, rifampicin, saquinavir, sulfonamides, tetracyclines, and TMP-SMX. [35, 36, 37]
Sweet syndrome
Causative agents include all-trans-retinoic acid, celecoxib, granulocyte colony-stimulating factor (G-CSF), nitrofurantoin, oral contraceptives, tetracyclines, and TMP-SMX.
Toxic epidermal necrolysis
Causative agents include alfuzosin, allopurinol, anticonvulsants, aspirin/NSAIDs, sulfadoxine-pyrimethamine, isoniazid, lamotrigine, lansoprazole, letrozole, penicillins, phenytoin, prazosin, sulfonamides, tetracyclines, thalidomide, TMP-SMX, and vancomycin.
Urticaria
Causative agents include ACE inhibitors, alendronate, aspirin/NSAIDs, blood products, cephalosporins, cetirizine, clopidogrel, dextran, didanosine, infliximab, inhaled steroids, nelfinavir, opiates, penicillin, peptide hormones, polymyxin, PPIs, radiologic contrast material, ranitidine, tetracycline, vaccines, and zidovudine.
Vasculitis
Causative agents include adalimumab, allopurinol, aspirin/NSAIDs, cimetidine, gold, hydralazine, indinavir, leflunomide, levofloxacin, minocycline, montelukast, penicillin, phenytoin, propylthiouracil, PPIs, quinolones, ramipril, sulfonamide, tetracycline, thiazides, and thioridazine.
Vesiculobullous (other)
Causative agents include ACE inhibitors, aspirin/NSAIDs, barbiturates, captopril, cephalosporins, entacapone, estrogen, furosemide, griseofulvin, influenza vaccine, penicillamine, penicillins, sertraline sulfonamides, and thiazides.
Photosensitivity reaction
Long-term use of voriconazole causes significantly increased photosensitivity, resulting in some patients developing squamous cell carcinoma (SCC) [38] and melanoma. [39] Studies have shown a dose-dependent increased risk for SCC: 5.6% with each 60-day exposure at a standard dose of 200 mg twice daily. At 5 years after transplantation, voriconazole conferred an absolute risk increase of 28% for SCC.
Psychotropic drugs associated with specific cutaneous reactions
Psychotropic agents associated with particular morphologic patterns include the following [40] :
-
Alopecia - Carbamazepine, fluoxetine, lamotrigine, lithium, gabapentin, and valproic acid
-
EM - Barbiturates, carbamazepine, diazepam overdose, fluoxetine, gabapentin, lithium plus trazodone concurrently, phenobarbital, risperidone, sertraline, and valproic acid
-
Morbilliform (exanthematous) - Alprazolam, barbiturates, bupropion, carbamazepine, chlorpromazine, desipramine, fluoxetine, lithium, maprotiline, nefazodone, risperidone, and trazodone
-
Photosensitivity - All antipsychotics, barbiturates, carbamazepine, chlorpromazine, doxepin, imipramine, thioridazine, and valproic acid
-
Pigmentation - Amitriptyline, carbamazepine, chlorpromazine, clozapine, diazepam following dermabrasion, gabapentin, haloperidol, lamotrigine, perphenazine, and thioridazine
-
Urticaria - Bupropion, carbamazepine, chlordiazepoxide, fluoxetine, imipramine, lamotrigine, lithium, paroxetine, and trazodone
-
Vasculitis - Fluoxetine, maprotiline, paroxetine, and trazodone
Chemotherapeutic drugs associated with specific cutaneous reactions
The following is a list of chemotherapeutic agents associated with particular morphologic patterns of cutaneous reactions.
Acneiform
Associated agents include cetuximab, [41] dactinomycin, erlotinib, [41] fluoxymesterone, gefitinib, medroxyprogesterone, and vinblastine. [42]
Acral erythema (erythrodysesthesia)
Associated agents include capecitabine, cisplatin, clofarabine, cyclophosphamide, cytarabine, docetaxel, doxorubicin, fluorouracil, gemcitabine, MTX, tegafur, and vinorelbine.
Alopecia
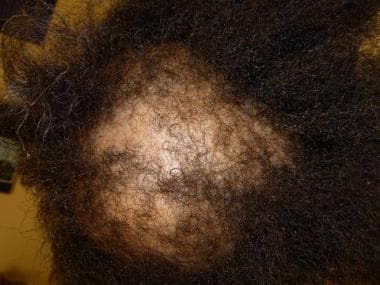
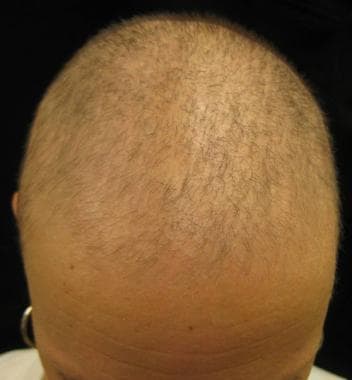
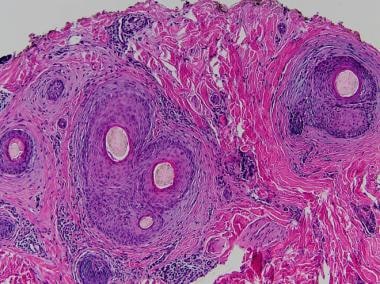
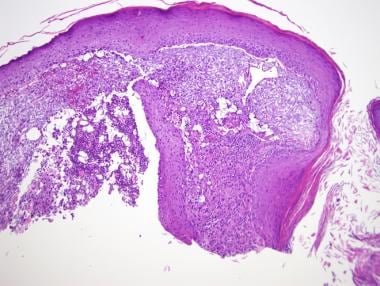
All classes of chemotherapeutic agents are associated with alopecia (see the first image below). Commonly associations include alkylating agents, anthracyclines, bleomycin, doxorubicin, hydroxyurea, MTX, mitomycin, mitoxantrone, vinblastine, and vincristine. Coadministration of busulfan and cyclophosphamide can cause permanent hair loss. Nilotinib is a potent and selective bcr-abl kinase inhibitor used to treat imatinib-resistant chronic myeloid leukemia. Clinically, pink/fleshy perifollicular papules with diffuse alopecia may be seen (see the second image below), without follicular dropout. Histologically, it can demonstrate scarring or nonscarring alopecia with mixed features (see the third image below). [43, 44]
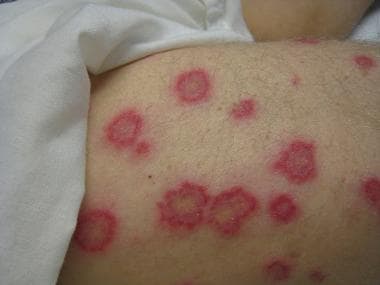
Erythema multiforme
Associated agents include busulfan, chlorambucil, cyclophosphamide, diethylstilbestrol (DES), etoposide, hydroxyurea, mechlorethamine, MTX, mitomycin C, mitotane, paclitaxel, and suramin.
Erythema nodosum
Associated agents include busulfan, DES, and imatinib.
Fixed drug eruptions
Associated agents include dacarbazine, hydroxyurea, paclitaxel, and procarbazine.
Hyperpigmentation
Associated agents include bischloroethylnitrosourea (BCNU; carmustine), bleomycin, busulfan, brequinar, cisplatin, cyclophosphamide, dactinomycin, daunorubicin, docetaxel, doxorubicin, fluorouracil, fotemustine, hydroxyurea, ifosfamide, MTX, mithramycin, mitoxantrone, nitrogen mustard, procarbazine, tegafur, thiotepa, and vinorelbine.
Lichenoid
Associated agents include hydroxyurea, imatinib, and tegafur.
Lupus
Associated agents include aminoglutethimide, DES, hydroxyurea, leuprolide, and tegafur.
Morbilliform (exanthematous)
Associated agents include bleomycin, carboplatin, cis-dichloro-trans-dihydroxy-bis-isopropylamine platinum (CHIP), chlorambucil, cytarabine, docetaxel, DES, doxorubicin, etoposide, 5-FU, hydroxyurea, MTX, mitomycin C, mitotane, mitoxantrone, paclitaxel, pentostatin, procarbazine, suramin, and thiotepa.
Toxic epidermal necrolysis
Associated agents include asparaginase, bleomycin, chlorambucil, cladribine, cytarabine, doxorubicin, 5-FU, MTX, plicamycin, procarbazine, and suramin.
Urticaria
Associated agents include amsacrine, bleomycin, busulfan, carboplatin, chlorambucil, cisplatin, cyclophosphamide, cytarabine, daunorubicin, diaziquone, didemnin, DES, docetaxel, doxorubicin, epirubicin, etoposide, 5-FU, mechlorethamine, melphalan, MTX, mitomycin C, mitotane, mitoxantrone, paclitaxel, pentostatin, procarbazine, teniposide, thiotepa, trimetrexate, vincristine, and zinostatin. [45]
Vasculitis
Associated agents include busulfan, cyclophosphamide, cytarabine, hexamethylene bisacetamide (HMBA), hydroxyurea, imatinib, levamisole, 6-mercaptopurine (MP), MTX, mitoxantrone, rituximab, and tamoxifen.
Cutaneous reactions caused by targeted chemotherapy agents
The following is a list of cutaneous reactions to targeted chemotherapy agents.
Epidermal growth factor receptor inhibitors
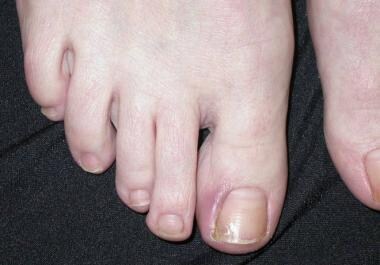
These agents (eg, gefitinib, cetuximab, erlotinib [46] ) can give rise to abnormal scalp, face hair, or eyelash growth; anaphylactic infusion reaction (cetuximab); papules and annular plaques; paronychia with or without pyogenic granulomas; telangiectasias; and xerosis. [47] (See the images below.)
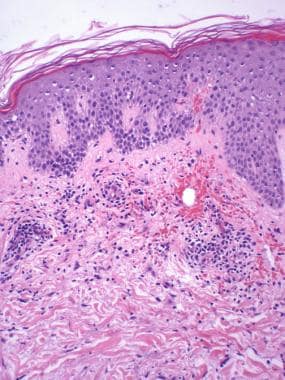 Superficial and middermal perivascular infiltrate of lymphocytes and eosinophils. Foci of extravasation of erythrocytes.
Superficial and middermal perivascular infiltrate of lymphocytes and eosinophils. Foci of extravasation of erythrocytes.
Sorafenib
Sorafenib, a multikinase inhibitor, can cause hand-foot skin reaction, facial and scalp eruption, scalp dysesthesia, subungual splinter hemorrhages, alopecia, body hair loss, stomatitis, nipple hyperkeratosis or pain, and eruptive facial cysts. [48]
Vemurafenib
Vemurafenib is a systemic medication approved by the Food and Drug Administration (FDA) for the treatment of metastatic melanoma. It selectively targets a specific BRAF mutation, V600E, in melanoma cells that allows unchecked proliferation of malignant cells. An unintended consequence of this medication has been the development of SCCs and keratoacanthomas in approximately one fourth of patients receiving the drug. The development of nonmalignant milia in a patient treated with vemurafenib has been reported. [49] (See the images below.)
Ipilimumab and vemurafenib each improve the overall survival of patients with metastatic melanoma. Patients with stage IV melanoma harboring a BRAF V600E mutation treated with vemurafenib after receiving ipilimumab can develop a pruritic grade 3 (severe) maculopapular reaction within 6-8 days after the start of treatment with vemurafenib. [50]
Tamoxifen
Tamoxifen, an antiestrogenic agent, has been widely used as adjuvant hormonal therapy in the treatment of breast cancer. Distinctive cutaneous eruptions present clinically as papules and plaques and histopathologically are characterized by squamous metaplasia of eccrine ductal epithelium. The condition has varied etiologies and can occur as a drug reaction, with chemotherapeutic drugs being frequently implicated. [51]
Cytokine agents causing cutaneous reactions
Cutaneous reactions to cytokine therapy include the following [52] :
-
Erythropoietin - Abnormal hair growth, localized rash, palpebral edema, and widespread eczema
-
G-CSF - Exacerbation of preexisting psoriasis, leukocytoclastic, localized erythema, localized pruritus, Sweet syndrome, and vasculitis
-
Granulocyte macrophage colony-stimulating factor (GM-CSF) - Alopecia, epidermolysis, exacerbation of vasculitis, exfoliative dermatitis, flushing, localized erythema, localized wheals, maculopapular eruptions, pruritus, purpura, and urticaria
-
IFN alfa - Alopecia, anasarca, cutaneous vascular lesions, eosinophilic fasciitis, exacerbation of preexisting herpes labialis, facial erythema, fixed drug eruption, hyperpigmentation, nummular eczema, paraneoplastic pemphigus, pruritus, psoriasis, sarcoidosis, SLE, urticaria, and xerostomia
-
IFN beta - Fatal pemphigus vulgaris (when used in combination with interleukin (IL)-2, localized reactions (common), and urticaria
-
IFN gamma - Increased relapses in melanoma and localized inflammation
-
IL-1α - Mucositis, phlebitis, Shwartzman reaction, and xerostomia
-
IL-1β - Erythema at surgical wound sites, phlebitis, and rash
-
IL-2 - Blisters, cutaneous ulcers, desquamation, erythema, erythema nodosum, erythroderma, exacerbation of autoimmune skin disorders, flushing, hypersensitivity to iodine contrast material, necrosis, pruritus, telogen effluvium, TEN, and urticaria
-
IL-3 - Facial flushing, hemorrhagic rash, thrombophlebitis, and urticaria
-
IL-4 - Facial and peripheral edema, Grover disease, and papular rash
-
IL-6 - Diffuse erythematous scaling macules and papules
-
TNF-α antagonists can also cause Sweet-like hypersensitivity reactions and neutrophilic eccrine hidradenitis in addition to pustular folliculitis, psoriasis, interface dermatitis, lupus, vasculitis, and palmoplantar pustulosis [53]
-
IL-12 and IL-23 monoclonal antibodies [54] - Injection-site reactions
Epidemiology
In the United States, drug eruptions occur in approximately 2-5% of inpatients and in more than 1% of outpatients. Internationally, drug eruptions occur in approximately 2-3% of inpatients.
Elderly patients have an increased incidence of adverse drug reactions. Adverse cutaneous reactions to drugs are more common in women than in men.
Prognosis
Most drug eruptions are mild and self-limited, and they typically resolve after the offending agent has been discontinued. Even after the responsible agent is discontinued, however, drug eruptions may be slow to clear or may worsen over the following few days. The degree of eosinophilia is predictive of the severity of the drug eruption. [55, 56] The time required for total clearing may be 1-2 weeks or longer.
Severe and potentially life-threatening eruptions occur in approximately 1 in 1000 hospital patients. Drug therapy complications account for roughly 19% of adverse events in hospitalized patients, making them the most common type; they account for 3% of disabling injuries during hospitalization. [57] Mortality figures for EM major are significantly higher. SJS has a mortality of less than 5%, whereas the mortality for TEN approaches 20-30%; most patients die from sepsis.
Patients with exanthematous eruptions should be counseled to expect mild desquamation as the rash resolves.
Patients with hypersensitivity syndrome are at risk of becoming hypothyroid, usually within the first 4-12 weeks after the reaction.
The prognosis for patients with TEN is guarded. Scarring, blindness, and death are possible.
Patient Education
If the responsible drug is identified, the patient should be advised to avoid that drug in the future. The medical record should be clearly labeled. Patients should be encouraged to carry a card or some other form of emergency identification in their wallets that lists drug allergies or intolerances, especially if they have had a severe reaction.
Patients should be educated about drugs that are cross-reactive and about drugs that must be avoided. For example, penicillin allergy reactions have cross-reactivity with cephalosporins, phenytoin hypersensitivity syndrome has cross-reactivity with phenobarbital and carbamazepine, and sulfonamide reactions cross-react with other sulfa-containing drugs.
-
Morbilliform drug eruption.
-
Warfarin necrosis involving leg.
-
Toxic epidermal necrolysis.
-
Stevens-Johnson syndrome.
-
Erythroderma.
-
Erythema multiforme.
-
Fixed drug eruption.
-
Fixed drug eruption involving penis.
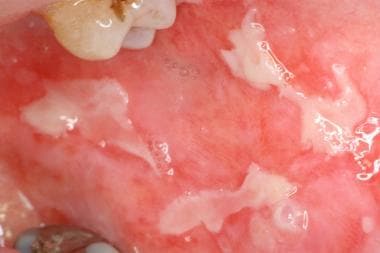
-
Oral ulcerations in patient receiving cytotoxic therapy.
-
Phototoxic reaction after use of tanning booth. Note sharp cutoff where clothing blocked exposure.
-
Vasculitic reaction on legs.
-
Lichen planus on neck.
-
Steroid acne. Note pustules and absence of comedones.
-
Drug reaction to hydroxychloroquine.
-
Urticaria.
-
Erythema nodosum.
-
Confluent necrosis of epidermis in toxic epidermal necrolysis.
-
Perivascular mixed inflammatory infiltrate with eosinophils characteristic of drug-induced urticaria.
-
Biopsy of pseudoporphyria shows subepidermal blister with little to no inflammation.
-
Confluent necrosis of epidermis in toxic epidermal necrolysis.
-
Superficial perivascular inflammatory infiltrate with numerous eosinophils characteristic of exanthematous drug eruption.
-
Target lesions of erythema multiforme.
-
Papules and annular plaques.
-
Superficial and middermal perivascular infiltrate of lymphocytes and eosinophils. Foci of extravasation of erythrocytes.
-
Numerous milia in patient treated with vemurafenib.
-
Dilated infundibular cyst.
-
Paronychia.
-
Male-pattern diffuse hair loss.
-
Pink/fleshy perifollicular papules with diffuse alopecia.
-
Horizontal section shows perifollicular fibrosis consistent with scarring alopecia.