Background
Aplasia cutis congenita (ACC) [1, 2] is a heterogenous group of disorders characterized by the absence of a portion of skin in a localized or widespread area at birth. First reported by Cordon in 1767, ACC most commonly manifests as a solitary defect but may sometimes occur as multiple lesions. (See the images below.) Although most commonly seen on the scalp (at least 70% of cases), ACC can affect any part of the body, including the trunk and limbs.
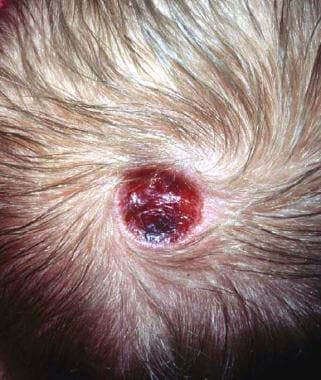
ACC lesions are noninflammatory and well-demarcated, varying widely in size. They may be circular, oval, linear, or stellate in configuration. ACC occurring in a blaschkoid distribution has also been reported. [3, 4] At birth, the lesions may have already healed with scarring or may remain superficially eroded to deeply ulcerated, occasionally involving the dura or the meninges. Defects in the skin that form early in gestation may heal before delivery and appear as an atrophic membranous, [5] bullous, [6] or parchmentlike scar with associated alopecia, whereas less mature defects present as ulcerations. The membranous type of ACC is most common.
Most ACC lesions occur on the scalp vertex just lateral to the midline, but defects may also occur on the face, trunk, or limbs, sometimes symmetrically. The defect may involve only the epidermis and upper dermis, resulting in minimal alopecic scarring, or it may extend into the deep dermis, subcutaneous tissue, or (rarely) the periosteum, skull, and dura. Large, irregular ACC lesions are suggestive of extension to deeper structures.
Although ACC is most often a benign isolated defect, it can be associated with other physical anomalies or malformation syndromes. [7] (See Pathophysiology.)
Pathophysiology
The exact pathophysiology of ACC is unclear. The most commonly accepted theory focuses on the tension that prevents the skin from converging during development in utero. Proposed mechanisms include intrauterine trauma, vascular compromise, infection, and medications. It has been theorized that stellate or angulated lesions in particular result from vascular abnormalities or intrauterine ischemia.
ACC is typically sporadic; however, autosomal dominant and, less commonly, autosomal recessive cases have also been reported. Mutations in the ribosomal GTPase BMS1 have been identified as one cause of autosomal dominant ACC. [8] Familial ACC on the scalp is generally nonmembranous, whereas membranous ACC of the scalp is usually sporadic. [9]
Research has shown that dominant-negative mutations in the genes KCTD1 and KCTD15 cause ACC through loss of function of KCTD1/KCTD15 complexes in cranial neural crest cells. [10] This loss of function hinders formation of normal midline cranial sutures and the overlying skin.
Classification
Frieden [11] created a classification system for ACC that consisted of nine groups, defined on the basis of the number and location of the lesions and the presence or absence of associated malformations.
Group 1
This is ACC of the scalp without multiple anomalies. [12] Nearly 86% of all solitary lesions occur on the scalp. A collar of hair is often seen around the defect, particularly with membranous ACC. It can be autosomal dominant [13] or sporadic. [14]
Group 2
This is ACC of the scalp with limb anomalies. [15] Adams-Oliver syndrome [16, 17, 18] is a distinct disorder in which distal limb-reduction abnormalities are found in association with solitary midline scalp defects. This syndrome exhibits both autosomal dominant and autosomal recessive patterns of inheritance. Mutations in EOGT and DOCK6 have been identified as causes of autosomal recessive Adams-Oliver syndrome, and mutations in DLL4, ARHGAP31, RBPJ, and NOTCH1 have been observed in autosomal dominant forms of the syndrome. [19, 20, 21, 22, 23, 24]
In group 2 ACC, the scalp lesions tend to be large. The most common limb malformation is hypoplastic or absent distal phalanges, but the severity of limb anomalies ranges from minor defects (eg, an absent nail or a broad fingertip) to more severe involvement. Limb anomalies are usually asymmetric and more commonly involve the lower extremities. [25] Other anomalies may include cutis marmorata telangiectatica congenita, hemangiomas, cranial arteriovenous malformation (AVM), congenital heart defects, skin tags, supernumerary nipples, and woolly hair.
Group 3
This is ACC of the scalp with epidermal and sebaceous (organoid) nevi [26, 27] that also involve the scalp, usually adjacent to the ACC. Some patients have also had ophthalmic and neurologic findings typical of epidermal nevus syndrome, including seizures, intellectual disability, corneal opacities, and eyelid colobomas. The term SCALP syndrome may be used as a label for the constellation of nevus sebaceus, central nervous system (CNS) malformations, ACC, limbal dermoid, and pigmented nevus. [28, 26] Inheritance is sporadic.
Group 4
This is ACC overlying deeper embryologic malformations [29, 30, 31, 32] (eg, meningomyelocele, porencephaly, leptomeningeal angiomatosis, cranial stenosis, spinal dysraphism, gastroschisis, and omphalocele). A hair collar is often present in the scalp lesions overlying neural tube defects. Intracranial AVMs and arteriovenous fistulas (AVFs) have also been reported in association with scalp ACC in rare cases. [33] The inheritance pattern in this group varies with the associated underlying condition. There is often a need for repair of abdominal-wall defects with this type of ACC. [34]
Group 5
This is ACC associated with fetus papyraceus or placental infarct. [35, 36, 37, 38, 39, 40, 41] Fetus papyraceus is found at the time of delivery and results from the death of a twin fetus in the late first or early second trimester. The surviving fetus is affected with extensive truncal and limb ACC in a linear or stellate configuration but is usually otherwise normal.
Group 6
This is ACC associated with epidermolysis bullosa (EB), [42, 43] , also referred to as Bart syndrome. ACC can be seen with any type of EB (simplex, junctional, or dystrophic). Many reports describe ACC in this setting, usually on the lower extremities. A subgroup includes the association of pyloric or duodenal atresia, ureteral stenosis, renal abnormalities, craniofacial abnormalities, nail dystrophy, and ACC.
Group 7
This is ACC localized to the extremities without EB. [44, 45, 46, 47] At least two families have been reported in which multiple members have had extensive ACC on the pretibial lower extremities and the dorsal aspects of the hands and the feet.
Group 8
This is ACC due to teratogens. A few cases of ACC have been linked to intrauterine infection with herpes simplex virus (HSV) or varicella zoster virus (VZV) or to exposure to methimazole [48, 49, 50] in the treatment of maternal thyrotoxicosis during pregnancy. Imperforate anus has been associated with methimazole or carbimazole exposure during gestation.
Group 9
This is ACC associated with malformation syndromes. [51, 52] ACC has been reported as a characteristic in many syndromes, [53, 54, 55, 56] including the following:
-
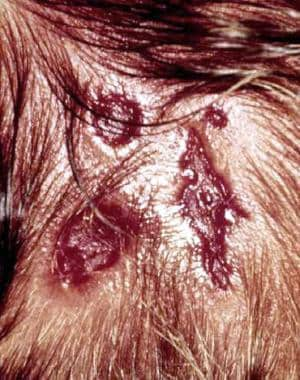
Trisomy 13 (Patau syndrome) with large membranous scalp defects
-
4p– (Wolf-Hirschhorn) syndrome with midline scalp defects
-
Setleis syndrome with bitemporal aplasia cutis congenita and abnormal eyelashes
-
Johanson-Blizzard syndrome with stellate scalp defects
-
Focal dermal hypoplasia (Goltz syndrome)
-
Amniotic band disruption complex
-
Oculocerebrocutaneous (Delleman) syndrome
-
Scalp-ear-nipple (Finlay-Mark) syndrome [57]
-
Kabuki syndrome [58]
-
46XY gonadal dysgenesis
Reticulolinear ACC on the face and neck is a distinctive cutaneous manifestation in several syndromes linked to band Xp22.
Etiology
No single unifying theory can account for all lesions of ACC. Because this condition is the phenotypic result of more than one disease process, it is likely that more than one mechanism is involved. Mechanisms include the following:
-
Genetic factors
-
Teratogens (eg, methimazole, carbimazole, misoprostol, valproic acid)
-
Compromised vasculature to the skin
-
Infections
-
Neural tube defects
-
Trauma
Of particular note is the association of fetus papyraceus with bilaterally symmetric ACC.
The proximity of scalp ACC to the scalp hair whorl, which is thought to be the point of maximum tensile force during rapid brain growth, led to the hypothesis that tension-induced disruption of the overlying skin occurs at 10-15 weeks' gestation, when hair direction, patterning, and rapid brain growth occur. This may also explain the increased incidence of ACC on the vertex scalp.
Early rupture of the amniotic membranes, forming amniotic bands, has appeared to be the cause of ACC in several cases.
The bullous or membranous variants of ACC reveal a distinct histologic pattern identical to those noted in encephaloceles and meningoceles. This finding supports the hypothesis that these types of ACC may represent the forme fruste of a neural tube closure defect.
Epidemiology
US and international statistics
ACC is an uncommon anomaly of newborns. Hundreds of cases of ACC have been reported since its initial description, but because of significant underreporting of this generally benign disorder, the precise frequency has not been established. For example, one estimate of incidence cited a figure of approximately three cases in 10,000 births, whereas another cited a figure of 0.3%. [59] A population-based study using data from 28 EUROCAT registries in 16 European countries reported an overall prevalence of 5.1 per 100,000 for the period from 1998 to 2017. [7]
Age-, sex-, and race-related demographics
ACC lesions are present at birth. Unless associated with an X-linked malformation syndrome, ACC has not been shown to have any sexual predilection. No racial predilection has been established.
Prognosis
The prognosis for patients with ACC is usually excellent. If the defect is small, recovery is uneventful, with gradual epithelialization and formation of a hairless, atrophic scar over several weeks. For patients treated conservatively, the average length of recovery is 27.9 days. [60] Small underlying bony defects usually close spontaneously during the first year of life. Surgical repair of large or multiple scalp defects, either with excision and primary closure (if feasible) or with the use of tissue expanders and rotation of a flap, may be considered. Truncal and limb defects, despite their large size, usually epithelialize and form atrophic scars, which can be revised later if necessary.
When ACC is associated with other anomalies, the prognosis depends on the severity of those anomalies. Underlying or associated defects may significantly affect mortality and morbidity. Full-thickness defects of the scalp, skull, and dura are associated with a mortality exceeding 50%. Even large defects on areas other than the scalp usually heal well with conservative skin care (eg, topical antibiotic ointment). The rare larger scalp defects are prone to hemorrhagic and infectious complications, placing patients at risk for death. Extensive ACC of the scalp may be associated with an increased risk of sagittal sinus thrombosis. For these reasons, surgical intervention may be required for large full-thickness scalp defects.
Patient Education
Genetic counseling is advised for parents of affected individuals if there is a strong family history of ACC or if the affected children have ACC in association with a malformation syndrome.
-
Aplasia cutis congenita on the scalp (most common location) shortly after birth.
-
Triplet areas of aplasia cutis congenita are common in infants with trisomy 13.
-
This area of healed aplasia cutis congenita is located in an area of nevus flammeus. Note the collarette of coarser hair at the margin of the defect.
-
Extensive aplasia cutis congenita on the scalp, extending down to the skull.
-
Bilateral involvement of the lower extremities in aplasia cutis congenita associated with fetus papyraceous.