Practice Essentials
Thrombotic thrombocytopenic purpura (TTP) is a rare blood disorder characterized by clotting in small blood vessels (thromboses), resulting in a low platelet count. [1, 2] In its full-blown form, the disease consists of the following pentad:
-
Microangiopathic hemolytic anemia
-
Thrombocytopenic purpura
-
Neurologic abnormalities
-
Fever
-
Kidney disease
To make an accurate diagnosis, the clinician must recognize the similarity between TTP and hemolytic-uremic syndrome (HUS). [3] In addition to HUS, the differential diagnosis includes immune thrombocytopenia (ITP) and disseminated intravascular coagulation (DIC), two entities with very different modes of therapy (see the image below).
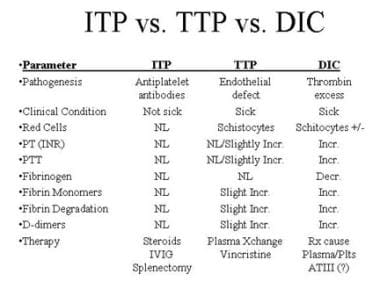 Differential diagnosis of immune thrombocytopenia (ITP), thrombotic thrombocytopenic purpura (TTP), and disseminated intravascular coagulation (DIC).
Differential diagnosis of immune thrombocytopenia (ITP), thrombotic thrombocytopenic purpura (TTP), and disseminated intravascular coagulation (DIC).
Secondary TTP has been associated with the use of certain drugs, including chemotherapy drugs such as gemcitabine and mitomycin and antiplatelet agents such as clopidogrel and ticlopidine. [4] If secondary TTP is suspected, the offending drug should be discontinued.
Signs and symptoms
TTP can affect any organ system, but involvement of the peripheral blood, the central nervous system, and the kidneys causes the clinical manifestations. Patients with TTP typically report an acute or subacute onset of symptoms related to neurologic dysfunction, anemia, or thrombocytopenia, as follows:
-
Neurologic manifestations include alteration in mental status, seizures, hemiplegia, paresthesias, visual disturbance, and aphasia
-
Fatigue may accompany the anemia
-
Severe bleeding from thrombocytopenia is unusual, although petechiae are common
See Presentation for more detail.
Diagnosis
Laboratory studies for suspected TTP include a CBC, platelet count, peripheral blood smear, coagulation studies, BUN and creatinine, and serum bilirubin and lactate dehydrogenase.
Most sporadic cases of TTP appear to be associated with severe deficiency of ADAMTS13 activity due to autoantibodies against this protease. [5, 6, 7] Measuring ADAMTS13 activity level may aid in diagnosis.
Imaging studies and biopsies are not required for diagnosis.
See Workup for more detail.
Management
The therapy of choice for TTP is plasma exchange with fresh frozen plasma. Because only 20-30% of patients present with the classic pentad, initiating total plasma exchange is justified by the presence of microangiopathic hemolytic anemia (schistocytes, elevated LDH, and indirect hyperbilirubinemia) and thrombocytopenia in the absence of other obvious causes (DIC, malignant hypertension).
Octaplas (Octapharma) is a sterile, frozen solution of pooled human plasma from several donors. It is a viable alternative to single-donor plasma, and it is treated with a solvent detergent process, which reduces the risk of infection. [8]
Caplacizumab (Cablivi), a nanobody that targets von Willebrand factor (vWF), is approved for treatment of acquired thrombotic thrombocytopenic purpura (aTTP) in combination with plasma exchange and immunosuppressive therapy. It has been shown to reduce time to platelet count response and also to reduce aTTP-related death, recurrence, or major thromboembolic events. [9]
A recombinant ADAMTS13 (Adzynma) was approved by the FDA in 2023 for prophylactic or on-demand enzyme replacement therapy in adults and children with congenital thrombotic thrombocytopenic purpura (cTTP).
In patients with TTP refractory to plasma exchange, using cryopoor plasma (or cryosupernatant) has sometimes led to a response. This is fresh frozen plasma that has had the cryoprecipitate removed and is thus depleted of high-molecular-weight von Willebrand multimers, which have a pathogenic role in TTP.
Corticosteroids may also be used in refractory TTP. Rituximab, although not approved for use in TTP, is increasingly recommended for refractory cases.
See Treatment and Medication for more detail.
Pathogenesis
TTP can affect any organ system, but involvement of the peripheral blood, the central nervous system, and the kidneys causes the clinical manifestations. The classic histologic lesion is one of bland thrombi in the microvasculature of affected organs. These thrombi consist predominantly of platelets, with little fibrin and red cells compared with thrombi that occur secondary to intravascular coagulation.
Patients with TTP have unusually large multimers of von Willebrand factor (vWF) in their plasma, and they have functional deficiency of a plasma protease that is responsible for the breakdown of these ultralarge vWF multimers. This protease has been isolated and cloned and is designated ADAMTS13 (A Disintegrinlike And Metalloprotease with ThromboSpondin type 1 motif 13). [10] The accumulation of ultralarge vWF multimers on the endothelial surface results in platelet aggregation and eventually thrombus formation. [2]
In more than 95% of cases, TTP is an acquired disorder that is due to autoantibodies that inhibit plasma ADAMTS13 activity; this form is termed immune-mediated TTP. [11, 12] In the remainder of cases, TTP is an inherited disorder in which mutations in the ADAMTS13 gene result in severe deficiency of functional plasma ADAMTS13. [13, 5] Hereditary or congenital TTP, also called Upshaw‐Schulman syndrome, accounts for less than 5% of all TTP cases but may account for 25% to 50% of cases in some patient populations, such as young children and pregnant women. [11]
In addition to immune and congenital TTP, a third form of TTP has been tentatively identified, and termed unidentified TTP. In contrast to immune TTP, anti-ADAMTS13 IgG antibodies are lacking in unidentified TPP. Significantly, whereas in patients with immune TTP, ADAMTS13 circulates in plasma in an open configuration, which makes it available for autoantibodies to bind with it, in unidentified TPP ADAMTS13 circulates in closed conformation, as is typical of healthy persons. Compared with immune TTP, unidentified TTP is less likely to occur in women, it tends to occur in older individuals, and patients more often have associated cancers and less often have accompanying autoimmune diseases. [14, 15]
While ADAMTS13 deficiency seems a necessary etiologic factor, by itself it may not be sufficient to induce TTP. Endothelial activation, caused by endogenous or exogenous factors and affecting mainly microvascular cells, has been proposed as a "second hit" that triggers TTP. [16] Triggers for TTP include infections, such as COVID-19 [17] ; pregnancy; autimmune disease; hematopoietic stem cell transplantation; and drugs, such as mitomycin, cyclosporine, cisplatin, bleomycin, quinine, ticlopidine and alemtuzumab. [18] Whether the drugs and/or their metabolites have a direct effect on the vascular endothelium or whether alteration of the endothelial cells results in a neoantigen that leads to autoantibody formation remains unknown.
Although the signs and symptoms of TTP overlap with those of classic hemolytic-uremic syndrome (HUS), ADAMTS13 activity is normal in most patients with classic HUS. This suggests a differing pathogenesis of these closely related entities. [19]
Epidemiology
Exact incidence figures for the United States are not available, although TTP is thought to be rare. In one series, the frequency was approximately 1 in 50,000 hospital admissions. Over a 25-year period in the Sacramento, California region (population at risk, 1.2 million), at least 176 documented cases of TTP were reported. In another 1-year study, 20 institutions reported 115 patients with TTP.
Analysis of a French national registry found that the rate of TTP in France was 13 cases per million population. [7] The age-sex standardized incidence of TTP has been estimated at 2.2 cases per million population per year in the United Kingdom and 3.2 cases per million population per year in Saskatchewan, Canada. [20] A systematic review estimated that worldwide, the incidence rate of acute episodes of immune TTP was 1.81-3.93 per million persons per year. [21]
Mortality/morbidity
Untreated, TTP has a mortality rate of as high as 90%. With plasma exchange, the mortality rate is reduced to 10-20%.
Acute morbidities include ischemic events such as stroke, transient ischemic attacks, myocardial infarction and cardiac arrhythmia, bleeding, and azotemia. TTP during pregnancy may precipitate fetal loss. [22]
In general, survivors have no long-term sequelae, with the exception of residual neurologic deficits in a minority of patients. However, relapses are not uncommon, occurring in 13-36% of patients.
Racial, sexual, and age-related disparities
An ethnic predisposition to TTP is not established. In the larger series reported, a female predominance of approximately 2:1 has been noted.
In several large studies, the median age at TTP diagnosis is approximately 40 years. However, in the authors' series of 126 consecutive patients, the median age was 52 years. This stands in contrast with HUS; 90% of cases of HUS occur in children. Bouw et al have presented a review article of TTP in children. [23]
Prognosis
The overall response rate to plasma exchange is 75-90%.The early mortality rate is 10-20%.
Long-term survival depends largely on the presence or absence of serious underlying comorbidities such as cancer, HIV infection, or solid organ transplantation. In the authors' series of 126 patients, the estimated 10-year survival rate of patients without comorbid conditions was 82%, compared with a survival rate of 50% in those with comorbid conditions.
A clinical severity score, incorporating the presence or absence of neurologic symptoms, creatinine, platelet count, and hemoglobin, was shown to be predictive of 30-day mortality in the authors' retrospective analysis. The absence of fever and a higher creatinine level was associated with a higher rate of relapse. However, upon further analysis of a larger cohort of patients (as yet unpublished), these factors are no longer predictive.
A study by Staley et al of 73 patients with immune-mediated TTP found that the following findings on admission were associated with higher mortality [24] :
-
Prolonged activated partial thromboplastin time
-
High fibrinogen level
-
Elevated serum lactate dehydrogenase level
-
Elevated complement activation marker Bb
-
Elevated complement activation marker sC5b-9
Other findings predictive of higher mortality included the following [24] :
-
Failure to normalize platelet counts within 7 days
-
Failure to markedly reduce serum lactate dehydrogenase by day 5
-
Low total serum protein or albumin
-
High troponin levels prior to total plasma exchange
Shumak et al reported that more than one third of patients who survive an acute episode of TTP will have at least one relapse in the following 10 years. [25] French researchers found that patients who have had acute TTP are at increased risk for development of an autoimmune disorder—most often, systemic lupus erythematosus (SLE) or Sjögren syndrome—for as long as 12 years afterward. Risk was highest in patients who had anti–double-stranded (ds)DNA antibodies (hazard ratio [HR] 4.98) or anti-SSA antibodies (HR 9.98) at the time of TTP diagnosis. These researchers recommend prolonged follow-up to detect any autoimmune disorder early in its course. [26]
-
Differential diagnosis of immune thrombocytopenia (ITP), thrombotic thrombocytopenic purpura (TTP), and disseminated intravascular coagulation (DIC).

