Background
Altitude illness is a collective term for a group of syndromes that result from hypoxia. Acute mountain sickness (AMS) and high-altitude cerebral edema (HACE; see the image below) are manifestations of the cerebral pathophysiology of altitude illness, whereas high-altitude pulmonary edema (HAPE) is a manifestation of the pulmonary pathophysiology. Everyone traveling to altitude is at risk for altitude illness, regardless of age, level of physical fitness, prior medical history, or previous altitude experience.
High elevations are generally considered to be those above 1500 m (4800 ft). Moderate altitude refers to elevations of 2000-3500 m (6400-11,200 ft); many US ski resorts are at altitudes within this range. Although arterial oxygen saturation (SaO2) is well maintained at these altitudes, low partial pressure of oxygen (PO2) results in mild tissue hypoxia, and altitude illness is common.
Very high altitude refers to elevations of 3500-5600 m (11,200-18,000 ft). SaO2 is not maintained in this range, and extreme hypoxemia can occur during sleep, with exercise, or with illness. HACE and HAPE are most common at these altitudes.
Extreme altitude refers to elevations above 5600 m (18,000 ft). At these elevations, successful long-term acclimatization is not possible; in fact, deterioration ensues. To reach extreme altitude, individuals must progressively acclimatize themselves to intermediate altitudes.
Pathophysiology
Acclimatization
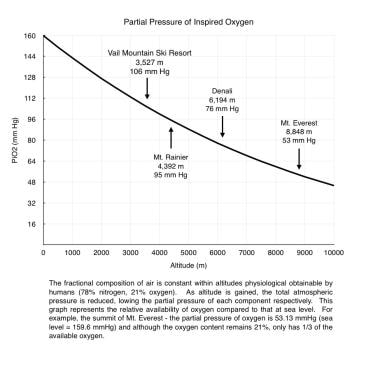
Hypoxia is the primary physiologic insult resulting from ascent to high altitude. The fraction of oxygen in the atmosphere remains constant (0.21) at all altitudes, but PO2 decreases along with barometric pressure on ascent to altitude (see the image below). The inspired partial pressure of oxygen (PiO2) is lower still because of water vapor pressure in the airways. At the altitude of the international airport at La Paz, Bolivia (4062 m; 13,327 ft), PiO2 is 98.18 mm Hg, which is equivalent to breathing 12.8% oxygen at sea level.
The response to hypoxia depends on both the magnitude and the rate of onset of hypoxia. The process of adjusting to hypoxia, termed acclimatization, is a series of compensatory changes in multiple organ systems over differing time courses that range from minutes to weeks. Whereas the fundamental process occurs within the metabolic machinery of the cell, acute physiologic responses are essential in allowing the cells time to adjust.
The most important immediate response of the body to hypoxia is an increase in minute ventilation, called the hypoxic ventilatory response (HVR), which is triggered by oxygen-sensing cells in the carotid bodies. Increased ventilation produces a higher alveolar PO2. Concurrently, a lowered alveolar partial rpessure of carbon dioxide (PCO2) produces a respiratory alkalosis, acting as a brake on the respiratory center of the brain and subsequently limiting further increases in ventilation.
Renal compensation, through excretion of bicarbonate ions, gradually brings blood pH back toward normal and allows a further increase in ventilation. This process, termed ventilatory acclimatization, requires approximately 4 days at a given altitude and is greatly enhanced by acetazolamide. Patients with inadequate carotid body response (genetic or acquired—eg, after surgery or radiation) or pulmonary or renal disease may have an insufficient HVR and thus may not adapt well to high altitude.
In addition to ventilatory changes, circulatory changes occur that increase delivery of oxygen to the tissues. Ascent to high altitude initially results in increased sympathetic activity, leading to a higher resting heart rate, greater cardiac output, and mildly increased blood pressure. Within minutes of exposure, the pulmonary circulation reacts to hypoxia with vasoconstriction. This may improve ventilation/perfusion (V/Q) matching and gas exchange, but the resulting pulmonary hypertension can lead to a number of pathologic syndromes at high altitude, including HAPE and altitude-related right heart failure.
Cerebral blood flow (CBF) increases immediately on ascent to high altitude, returning toward normal over about a week. The magnitude of the increase varies but averages 24% at 3810 m and is greater at higher altitude. It is believed that this flow increase is partially responsible for the headache of AMS. [1]
Hemoglobin concentration increases after ascent to high altitude, thereby improving the oxygen-carrying capacity of the blood. Initially, it increases as a result of hemoconcentration from reduced plasma volume secondary to altitude diuresis and fluid shifts. Subsequently, over days to weeks of hypoxia exposure, erythropoietin stimulates increased red blood cell production. In addition, the marked alkalosis of extreme altitude causes a leftward shift of the oxyhemoglobin dissociation curve, facilitating loading of the hemoglobin with oxygen within the pulmonary capillary bed.
Sleep architecture is altered at high altitude, with frequent arousals and nearly universal subjective reports of disturbed sleep. This disturbance generally improves after several nights at a constant altitude, though periodic breathing (Cheyne-Stokes respiration) remains common above 2700 m. The use of acetazolamide has been demonstrated to reduce the symptoms of high-altitude sleep disturbance.
Altitude-related cerebral pathology
The exact pathophysiology of AMS/HACE is unknown. The prevailing hypothesis has been that hypoxia elicits neurohumoral and hemodynamic responses in the brain that ultimately result in capillary leakage from microvascular beds and edema. Whether mild AMS or headache alone is actually due to brain edema remains an open question.
Studies using ultrasonographic assessment of optic nerve sheath diameter (ONSD) (see the image below), which has been shown to correlate with intracranial pressure (ICP), has demonstrated increased ONSD swelling in both AMS and HACE.
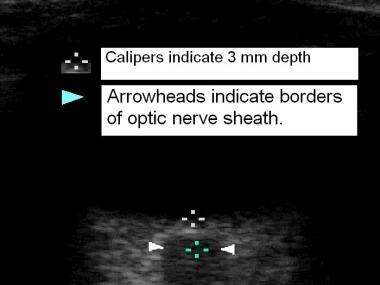 Ultrasonographic measurement of optic nerve sheath diameter. Top of field is cornea, bottom of field reveals retina, then optic nerve in lowest field. Images from Dr Peter Fagenholz et al.
Ultrasonographic measurement of optic nerve sheath diameter. Top of field is cornea, bottom of field reveals retina, then optic nerve in lowest field. Images from Dr Peter Fagenholz et al.
Magnetic resonance imaging (MRI) studies have demonstrated that the brain swells on ascent to altitude in both those with AMS and those without AMS, presumably from vasodilation. True edema, however, is detected only in severe AMS and HACE. Factors that might contribute to hydrostatic brain edema are multiple and include cerebral vasodilation, elevated cerebral capillary pressure, and impaired cerebral autoregulation, as well as alterations in the permeability of the blood-brain barrier through cytokine activation.
Susceptibility to AMS demonstrates great individual variability related to genetic differences. [2] Individual susceptibility is reproducible; a past history of AMS is the best predictor.
Etiology
Rapid ascent to altitudes above 2500 m can cause AMS. The risk of HACE or AMS increases with altitude.
Special attention should be paid to the elevation at which the person sleeps. Daytime climbs to higher elevations, with a return to lower altitudes for sleeping, are preferred.
Continued ascent despite symptoms of AMS is a major risk factor for developing HACE. At altitudes above 5000 m, ascents of as little as 200 m have been known to precipitate HACE in individuals with moderate AMS.
HACE frequently is seen secondary to HAPE, presumably because of rapidly worsening hypoxia, which is equivalent to continued ascent to higher altitudes.
Epidemiology
US and international statistics
The incidence of AMS in the United States varies according to the rate of ascent and the maximum altitude reached. In ski resorts at moderate altitude (2000-3500 m), the reported incidence has been in the range of 10-40%. Rapid ascent to approximately 4000 m has been associated with an incidence in the range of 60-70%.
Travelers flying to a high-altitude destination such as Lhasa, Tibet (3810 m; 12,500 ft), or La Paz, Bolivia (4062 m; 13,327 ft), can expect an AMS incidence of 25-35%. Those who hike above 4000 m (and ascend at a moderate pace) can expect a 25-50% incidence of AMS. HACE is estimated to occur in no more than 1% of persons traveling above 4000 m and in 1-3% of those with AMS.
Age-, sex-, and race-related demographics
In adults, age has a small effect on the incidence of high-altitude cerebral syndromes, with younger adults being slightly more susceptible than older ones. The occurrence rates in children are similar to those in adults.
No significant sex-related differences in frequency have been demonstrated. The incidence of AMS is not markedly affected by menstrual cycle phase and is the same for pregnant individuals as for nonpregnant ones.
No racial predilection is known.
Prognosis
The prognosis is excellent for AMS and for survivors of HACE. Once patients have completely recovered (ie, are fully asymptomatic), cautious reascent is acceptable. It is common for climbers to develop AMS, descend slightly, and then resume their ascent 1 or 2 days later, after full resolution of their symptoms.
The natural history of AMS varies with altitude, ascent rate, and other factors. In general, the illness is self-limiting, and symptoms improve slowly, typically resolving completely in 1-3 days without treatment. With continued ascent, however, AMS is very likely to worsen and is more likely to progress to HACE.
HACE may progress to stupor and coma over hours to days if untreated. Once coma has developed, the patient is more likely to die even if aggressive treatment is provided. Death is due to brain herniation. If descent is immediate and treatment is started promptly, the usual course of HACE is rapid, complete recovery; if treatment is delayed, recovery is slower. In rare cases, patients with either severe or prolonged HACE may have persistent neurologic deficits. Ataxia commonly persists for days to weeks and is often the last finding to resolve.
Patient Education
Patients should be educated regarding the value of staged ascents (see Prevention) and reminded of the golden rules of altitude illness, which are as follows:
-
If you feel unwell at altitude, it is altitude illness unless proved otherwise
-
If you have symptoms of altitude illness, go no higher
-
If your symptoms are worsening, if they do not improve with treatment, or if HACE or HAPE is present, descend immediately
For patient education resources, see Altitude Sickness (Mountain Sickness).
-
Partial pressure of inspired oxygen vs altitude.
-
Ultrasonographic measurement of optic nerve sheath diameter. Top of field is cornea, bottom of field reveals retina, then optic nerve in lowest field. Images from Dr Peter Fagenholz et al.
-
High-altitude cerebral edema (HACE). Image from Dr Peter Hackett.
-
Horse evacuation of nonambulatory altitude illness. Patient in Khumbu, Nepal. Image from Dr Peter Fagenholz.
-
Very ataxic man with high-altitude cerebral edema (HACE) at 4250 m being assisted toward Gamow bag.
-
Fully inflated Gamow bag in use.