Practice Essentials
Neonatal hypoglycemia, defined as a plasma glucose level of less than 30 mg/dL (1.65 mmol/L) in the first 24 hours of life and less than 45 mg/dL (2.5 mmol/L) thereafter, is the most common metabolic problem in newborns. Major long-term sequelae include neurologic damage resulting in intellectual disability, recurrent seizure activity, developmental delay, and personality disorders. Some evidence suggests that severe hypoglycemia may impair cardiovascular function.
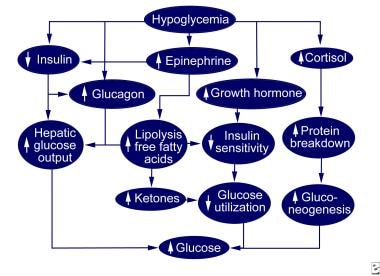
The image below depicts normal hypoglycemic counterregulation.
Signs and symptoms of neonatal hypoglycemia
Infants in the first or second day of life may be asymptomatic or may have life-threatening central nervous system (CNS) and cardiopulmonary disturbances. Symptoms can include the following:
-
Hypotonia
-
Lethargy, apathy
-
Poor feeding
-
Jitteriness, seizures
-
Congestive heart failure
-
Cyanosis
-
Apnea
-
Hypothermia
Clinical manifestations associated with activation of the autonomic nervous system include the following:
-
Anxiety, tremulousness
-
Diaphoresis
-
Tachycardia
-
Pallor
-
Hunger, nausea, and vomiting
Clinical manifestations of hypoglycorrhachia or neuroglycopenia include the following:
-
Headache
-
Mental confusion, staring, behavioral changes, difficulty concentrating
-
Visual disturbances (eg, decreased acuity, diplopia)
-
Dysarthria
-
Seizures
-
Ataxia, somnolence, coma
-
Stroke (hemiplegia, aphasia), paresthesias, dizziness, amnesia, decerebrate or decorticate posturing
See Clinical Presentation for more detail.
Diagnosis of neonatal hypoglycemia
Laboratory studies
These include the following:
-
Serum or plasma glucose levels
-
Serum insulin
-
Urine - Obtain a first-voided urine dipstick for ketones; send urine for organic acid analysis
-
Screening for metabolic errors - Electrospray ionization-tandem mass spectrometry in asymptomatic persons allows for earlier identification of clearly defined inborn errors of metabolism, including aminoacidemias, urea cycle disorders, organic acidurias, and fatty acid oxidation disorders
Angiography
The detection of adenomas by celiac angiography has had limited success. The chance of detecting a tumor blush must be balanced against the potential risk of causing vascular trauma in infants younger than 2 years.
See Workup for more detail.
Management
Hypoglycemia should be treated as soon as possible to prevent complications of neurologic damage. Early feeding of the newborn with breast milk or formula is encouraged.
For patients who cannot protect their airway or are unable to drink, nasogastric, intramuscular, intraosseous, or intravenous (IV) routes can be employed for the following drugs used to raise glucose levels: dextrose, glucagon, diazoxide, and octreotide. Start a 5% or 10% dextrose drip when hypoglycemia is recurrent.
Surgery
Surgical exploration usually is undertaken in severely affected neonates who are unresponsive to glucose and somatostatin therapy. Near-total resection of 85-90% of the pancreas is recommended for presumed congenital hyperinsulinism, which is most commonly associated with an abnormality of beta-cell regulation throughout the pancreas. Risks include the development of diabetes.
See Treatment and Medication for more detail.
Background
Hypoglycemia is the most common metabolic problem in neonates. In children, a blood glucose value of less than 40 mg/dL (2.2 mmol/L) represents hypoglycemia. A plasma glucose level of less than 30 mg/dL (1.65 mmol/L) in the first 24 hours of life and less than 45 mg/dL (2.5 mmol/L) thereafter constitutes hypoglycemia in the newborn.
Patients with hypoglycemia may be asymptomatic or may present with severe central nervous system (CNS) and cardiopulmonary disturbances. The most common clinical manifestations can include altered level of consciousness, seizure, vomiting, unresponsiveness, and lethargy. Any acutely ill child should be evaluated for hypoglycemia, especially when history reveals diminished oral intake. (See History and Physical Examination.)
Sustained or repetitive hypoglycemia in infants and children has a major impact on normal brain development and function. Evidence suggests that hypoxemia and ischemia potentiate hypoglycemia, causing brain damage that may permanently impair neurologic development. (See Prognosis.)
Causes of hypoglycemia in neonates differ slightly from those in older infants and children. The causes in neonates include the following (see Etiology):
-
Inappropriate changes in hormone secretion
-
Inadequate substrate reserve in the form of hepatic glycogen
-
Inadequate muscle stores as a source of amino acids for gluconeogenesis
-
Inadequate lipid stores for the release of fatty acids
Hyperinsulinism, or persistent hyperinsulinemic hypoglycemia of infancy (PHHI), is the most common cause of hypoglycemia in the first 3 months of life. It is well recognized in infants of mothers with diabetes. (See Etiology.)
Causes of hypoglycemia found in all ages include gram-negative sepsis, endotoxin shock, and ingestions, including of salicylates, alcohol, hypoglycemic agents, or beta-adrenergic blocking agents.
Excluding insulin therapy, almost all hypoglycemia in childhood occurs during fasting. Postprandial hypoglycemia is rare in children in the absence of prior gastrointestinal (GI) surgery. Management efforts are directed toward the immediate normalization of glucose levels and the identification and treatment of the various causes. (See Treatment and Medications.)
Patient education
Provide genetic counseling for families with affected children, including information about a possible 25% risk of recurrence. Educate pregnant women with diabetes. [1]
Glucose metabolism
Normal blood glucose is very narrowly regulated, usually from 80-90 mg/dL (4.4-5 mmol/L). Glucose levels increase transiently after meals to 120-140 mg/dL (6.6-7.7 mmol/L). Feedback systems return the glucose concentration rapidly back to the preprandial level, usually within 2 hours after the last absorption of carbohydrates.
Insulin and glucagon are the important hormones in the immediate feedback control system of glucose. When blood glucose increases after a meal, the rate of insulin secretion increases and stimulates the liver to store glucose as glycogen. When cells (primarily liver and muscle) are saturated with glycogen, additional glucose is stored as fat.
When blood glucose levels fall, glucagon secretion functions to increase blood glucose levels by stimulating the liver to undergo glycogenolysis and release glucose back into the blood. (See the diagram below.)
In starvation, the liver maintains the glucose level via gluconeogenesis. Gluconeogenesis is the formation of glucose from amino acids and the glycerol portion of fat. Muscle provides a store of glycogen and muscle protein breaks down to amino acids, which are substrates utilized in gluconeogenesis in the liver. Circulating fatty acids are catabolized to ketones, acetoacetate, and B-hydroxybutyrate and can be used as auxiliary fuel by most tissues, including the brain.
The hypothalamus stimulates the sympathetic nervous system, and epinephrine is secreted by the adrenals, causing the further release of glucose from the liver. Over a period of hours to days of prolonged hypoglycemia, growth hormone and cortisol are secreted and decrease the rate of glucose utilization by most cells of the body.
In the newborn, serum glucose levels decline after birth until age 1-3 hours; then they spontaneously increase. Liver glycogen stores become rapidly depleted within hours of birth, and gluconeogenesis, primarily from alanine, can account for 10% of glucose turnover in the newborn infant by several hours of age.
Pathophysiology
A prospective cohort study by Hoermann et al found that in neonates in the report who were at risk for hypoglycemia, increased umbilical cord blood concentrations of catecholamine and metanephrine showed a correlation with the number of episodes of postnatal hypoglycemia. These findings were consistent with an animal model that pointed to an association between fetal catecholamines and neonatal beta-cell physiology, and that indicated “that perinatal stress or growth restriction is associated with subsequent neonatal hyperinsulinemic hypoglycemia.” [2]
Etiology
The causes of neonatal hypoglycemia include the following:
-
PHHI
-
Limited glycogen stores (eg, prematurity, intrauterine growth retardation)
-
Increased glucose use (eg, hyperthermia, polycythemia, sepsis, growth hormone deficiency)
-
Decreased glycogenolysis, gluconeogenesis, or use of alternate fuels (eg, inborn errors of metabolism, adrenal insufficiency)
-
Depleted glycogen stores (eg, asphyxia-perinatal stress, starvation)
With regard to the last item above, in ketotic hypoglycemia, easily depleted glycogen stores, in combination with inadequate production of glucose through gluconeogenesis, contribute to hypoglycemia. Thus, fatty acid oxygenation is required to provide substrate for gluconeogenesis and ketogenesis. Ketones, the byproduct of fatty acid metabolism, are found in urine and represent the starved state.
A study by Ogunyemi et al indicated that independent risk factors for neonatal hypoglycemia include macrosomia, cesarean section, lower gestational age, and treatment for chorioamnionitis. Small-for-gestational-age neonates are also at greater risk. The study included 318 neonates with hypoglycemia and 7955 controls. [3]
A retrospective study by Yamamoto et al indicated that in women with type 1 diabetes mellitus, large-for-gestational-age neonates have a 2.5-fold increased risk for hypoglycemia. The study looked at pregnancies in 161 women with type 1 diabetes. [4]
A study by Anwer et al indicated that the risk for neonatal hypoglycemia is 1.8-fold higher when maternal hyperglycemia occurs prior to delivery in mothers with gestational diabetes mellitus (GDM) that is being managed with medication. Such an increase was not found in the offspring of mothers with diet-controlled GDM. [5]
A retrospective study by Mitchell et al found that out of 175 infants born at less than 33 weeks’ gestation, 33.7% had hypoglycemia within the first 90 minutes after birth. The investigators also found that maternal hypertension increased the risk of neonatal hypoglycemia by about 3-fold; they suggested that beta blockers used in antihypertensive treatment may have contributed to the higher odds. In contrast, labor at the time of delivery was reported to reduce the likelihood of hypoglycemia. [6]
Causes of hypoglycemia in older infants, children, and teenagers include:
-
Poisonings/drugs (eg, ethanol, isoniazid, insulin, propranolol, salicylates, oral hypoglycemics, pentamidine, quinine, disopyramide, unripe ackee fruit, Vacor [rat poison]).
-
Liver disease (eg, Reye syndrome, hepatitis, cirrhosis, hepatoma)
-
Amino acid and organic acid disorders (eg, maple syrup urine disease, propionic acidemia, methylmalonic acidemia, tyrosinosis, glutaric aciduria, 3-hydroxy-3-methylglutaric aciduria)
-
Systemic disease (eg, sepsis, burns, cardiogenic shock, respiratory distress syndrome)
Hyperinsulinemia
Congenital hyperinsulinism is most commonly associated with an abnormality of beta-cell regulation throughout the pancreas. A focal disease, such as isolated islet adenoma, occasionally causes congenital hyperinsulinism.
Genetic defects have been delineated and now replace the older terms, such as nesidioblastosis, leucine-sensitive hypoglycemia, PHHI, and islet dysregulation syndrome. These defects are in the sulfonylurea receptor (SUR) and the beta-cell potassium adenosine triphosphate (ATP) channel gene located on the short arm of chromosome 11.
Drug-induced hyperinsulinism is secondary to surreptitious insulin administration or the use of oral hypoglycemic drugs. Exogenous administration of insulin is diagnosed with low serum levels of C-peptide. The sulfonylureas are commonly prescribed for adults; thus, they are available to children as unintentional ingestions. In these cases, hypoglycemia may persist for more than 24 hours. Diazoxide administration may be helpful by suppressing insulin secretion in severe cases.
Epidemiology
Occurrence in the United States
The overall incidence of symptomatic hypoglycemia in newborns varies from 1.3-3 per 1000 live births. Incidence varies with the definition, population, method and timing of feeding, and the type of glucose assay. Serum glucose levels are higher than whole blood values. The incidence of hypoglycemia is greater in high-risk neonatal groups (see History).
Early feeding decreases the incidence of hypoglycemia. Inborn errors of metabolism that lead to neonatal hypoglycemia are rare but can be screened for in infancy. [7] The incidences of these conditions are as follows:
-
Carbohydrate metabolism disorders (>1:10,000)
-
Fatty acid oxidation disorders (1:10,000)
-
Hereditary fructose intolerance (1:20,000 to 1:50,000)
-
Glycogen storage diseases (1:25,000)
-
Galactosemia (1:40,000)
-
Organic acidemias (1:50,000)
-
Phosphoenolpyruvate carboxykinase deficiency (rare)
-
Primary lactic acidosis (rare)
International occurrence
In a Japanese study, more than 80% of admissions from the nursery to the neonatal intensive care unit (NICU) after birth were due to apnea or hypoglycemia in neonates born at 35-36 weeks' gestation. [8]
Prognosis
Hypoglycemia is the most common metabolic problem in neonates. Still, the level or duration of hypoglycemia that is harmful to an infant's developing brain is not known. Major long-term sequelae include neurologic damage resulting in intellectual disability, recurrent seizure activity, developmental delay, and personality disorders.
However, a study by Shah et al found that in mid-childhood (age 9-10 years), rates of low educational achievement did not significantly differ between children who had been exposed to neonatal hypoglycemia and those who had not (45% vs 47%, respectively). The investigators also found a significantly lower likelihood that teachers would rate children in this age group who had been exposed to neonatal hypoglycemia “as being below or well below the curriculum level for reading,” compared with other students (24% vs 31%, respectively). [9]
Some evidence suggests that severe hypoglycemia may impair cardiovascular function.
Although the occipital lobes, which contain the primary visual cortex and several extrastriate visual areas, may be especially vulnerable to the effects of neonatal hypoglycemia, a literature review by Paudel et al found the evidence as to whether neonatal hypoglycemia negatively impacts eye and optic nerve development to be inconclusive. [10]
Remission of congenital hyperinsulinism generally does not occur, but the severity of the disease may decrease with time.
-
Normal hypoglycemic counterregulation.