Practice Essentials
Chlorine gas is a pulmonary irritant with intermediate water solubility that causes acute damage in the upper and lower respiratory tract. Occupational exposures constitute the highest risk for serious toxicity from high-concentration chlorine (see the image below). Mixing of chlorine bleach (sodium hypochlorite) with ammonia or acidic cleaning agents is a common source of household exposure. As with all poisons, the dose determines the toxicity. Exposure to low concentrations of chlorine for prolonged periods may have destructive effects, as may very short-term exposure to high concentrations. [1]
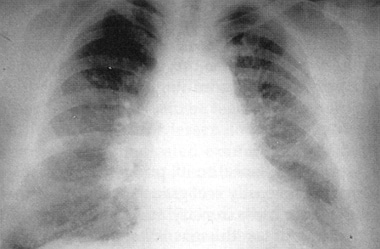 Chest radiograph of a 36-year-old chemical worker 2 hours postexposure to chlorine inhalant. She had severe resting dyspnea during the second hour, diffuse crackles/rhonchi on auscultation, and a partial pressure of oxygen of 32 mm Hg breathing room air. The radiograph shows diffuse pulmonary edema without significant cardiomegaly. Used with permission from Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. 1997: 256.
Chest radiograph of a 36-year-old chemical worker 2 hours postexposure to chlorine inhalant. She had severe resting dyspnea during the second hour, diffuse crackles/rhonchi on auscultation, and a partial pressure of oxygen of 32 mm Hg breathing room air. The radiograph shows diffuse pulmonary edema without significant cardiomegaly. Used with permission from Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. 1997: 256.
Signs and symptoms
Symptoms may vary depending on the degree of exposure. Exposure possibilities include acute low levels, acute high levels, and chronic low levels.
Low-level (3-5%, 1-15 ppm) acute exposure
Most poisonings fall into this category and are caused by household exposure to low-concentration cleaning products. Manifestations are as follows:
-
Eye tearing, nose and throat irritation
-
Sneezing
-
Excess salivation
-
General excitement or restlessness
High-level (20%, >30 ppm) acute exposure
In addition to the symptoms seen with low-level exposure, high-level exposure may result in the following:
-
Dyspnea - Upper airway swelling and obstruction may occur
-
Violent cough
-
Nausea and vomiting (with the odor of chlorine in emesis)
-
Lightheadedness
-
Headache
-
Chest pain or retrosternal burning
-
Muscle weakness
-
Abdominal discomfort
-
Dermatitis (with liquid exposure) - Corneal burns and ulcerations may occur from splash exposure to high-concentration chlorine products
-
Esophageal perforation
Chronic exposure
Manifestations of chronic exposure include the following:
-
Acne (chloracne)
-
Chest pain
-
Cough
-
Sore throat
-
Hemoptysis
Findings on physical examination may include the following:
-
Tachypnea
-
Cyanosis (most prevalent during exertion)
-
Tachycardia
-
Wheezing
-
Intercostal retractions
-
Decreased breath sounds
-
Rales (pulmonary edema)
-
Nasal flaring
-
Aphonia, stridor, or laryngeal edema
-
Ulceration or hemorrhage of the respiratory tract
-
Rhinorrhea
-
Lacrimation, salivation, and blepharospasm
-
Chloracne or tooth enamel corrosion (with chronic exposure)
-
Redness, erythema, and chemical burns to the skin from dose-dependent exposure to liquid
See Presentation for more detail.
Diagnosis
Studies in patients with significant exposure to chlorine gas may include the following:
-
Pulse oximetry
-
Serum electrolyte, blood urea nitrogen (BUN), and creatinine levels
-
Arterial blood gases
-
Chest radiography
-
Electrocardiogram
-
Computed tomography (CT) scan of the chest
-
Ventilation-perfusion scan
-
Pulmonary function testing
-
Laryngoscopy or bronchoscopy
Abnormalities include hypoxia (from bronchospasm or pulmonary edema) [2] and metabolic acidosis. The metabolic acidosis may be hyperchloremic (nonanion gap). Chest radiograph findings are frequently normal initially but later may show nonspecific abnormalities, pulmonary edema, pneumonitis, mediastinal free air, myocardial depression, [3] or signs of acute respiratory distress syndrome (ARDS).
See Workup for more detail.
Management
The most important aspect of treating patients exposed to chlorine gas is the provision of good supportive care, as follows:
-
Supplemental oxygen (humidified, if possible) as necessary
-
Fluid restriction and diuretics for impending pulmonary edema
-
Positive end-expiratory pressure (PEEP) in patients with noncardiogenic pulmonary edema
-
Beta2 agonists and other bronchodilators as necessary for bronchospasm
-
Nebulized lidocaine for analgesia and reduction of cough
-
Consider nebulized sodium bicarbonate
-
Consider inhaled or parenteral corticosteroids
-
Copious saline irrigation for skin or eye exposures
Consider admission and observation for the following patients, even if they are initially asymptomatic, as they are at increased risk for progression to respiratory failure:
-
Patients exposed to large concentrations in an enclosed environment
-
Patients with underlying respiratory or cardiovascular disease
-
Children
See Treatment and Medication for more detail.
Educate patients on the risks associated with the improper handling of chlorine pool chemicals and the improper mixing of household cleaning chemicals.
Background
As stated, chlorine gas is a pulmonary irritant with intermediate water solubility that causes acute damage in the upper and lower respiratory tract. Currently, occupational exposures constitute the highest risk for serious toxicity from high-concentration chlorine. Mixing of chlorine bleach (sodium hypochlorite) with ammonia or acidic cleaning agents is a common source of household exposure. See Etiology.
Chlorine gas has also been used repeatedly as a chemical weapon.3 It was officially introduced into the chemical warfare arsenal in 1915 at Ypres, Belgium. Accounts of chlorine attacks at Ypres describe an olive-green cloud rolling over the Allied positions, following the ground contours, and sinking into the trenches. Soldiers seeking safety in those trenches were overcome by the gas and experienced tearing eyes, vomiting, and difficulty breathing. They abandoned their trenches and suffered great losses from artillery and rifle fire.
An estimated 93,800 tons of chlorine gas were produced during World War I, with more than half produced by Germany. Total gas casualties in World War I were estimated at almost 1.3 million troops. Of the 70,552 American soldiers poisoned with various gases in World War I, 1843 were exposed to chlorine gas. [4] Chlorine was abandoned as a warfare agent when the use of gas masks was introduced and more effective compounds were created and deployed.
However, on at least three occasions in January and February 2007, insurgents in Iraq incorporated chlorine tanks in vehicle-borne improvised explosive device (VBIED) attacks. Most of the deaths from the attacks were caused by the explosion, but many people were treated and hospitalized for chlorine exposure. [5] It has also been reported that Islamic State militants used chlorine gas when attacking the northern Iraq city of Kirkuk in 2016. [6]
The Organization for the Prohibition of Chemical Weapons (OPCW) confirmed that in the Syrian civil war, which began in 2011, chlorine gas was used in numerous chemical attacks, affecting thousands of civilians. [7]
Pathophysiology
Chlorine is a greenish-yellow, noncombustible gas at room temperature and atmospheric pressure. Prolonged exposure to chlorine gas may occur because its moderate water solubility delays onset of upper airway symptoms for several minutes. In addition, chlorine gas is heavier than air in its pure form, causing it to remain near ground level and increasing exposure time.
The odor threshold for chlorine is approximately 0.3-0.5 parts per million (ppm); however, distinguishing toxic air levels from permissible air levels may be difficult until irritative symptoms develop. As the concentration of chlorine gas exposure increases, the severity of symptoms and rapidity of onset increase. The IDHL (immediately dangerous to life or health) is 10 ppm. Concentrations above 400 ppm are often fatal. [8]
Chlorine is moderately soluble in water and reacts in combination to form hypochlorous (HOCl) and hydrochloric (HCl) acids. Elemental chlorine and its derivatives, hydrochloric and hypochlorous acids, may cause biological injury. The chemical reactions of chlorine combining with water and the subsequent derivative reactions with HOCl and HCl are as follows:
a1) Cl2 + H2O ⇔ HCl (hydrochloric acid) + HOCL (hypochlorous acid) or
a2) Cl2 + H2O ⇔ 2 HCl + [O-] (nascent oxygen)
b) HOCl ⇔ HCl + [O-]
Chlorine gas, when mixed with ammonia, reacts to form chloramine gas. In the presence of water, chloramines decompose to ammonia and hypochlorous acid or hydrochloric acid. [9] Because of their high water solubility, chloramine exposures result in rapid symptom development. However, for mechanistic reasons that are not clear, chlorine 88 nitrogenous compounds result in less severe symptoms at onset. Because these initial symptoms are often mild, however, they may not prompt immediate retreat, thus resulting in prolonged exposure, with pulmonary and ocular symptoms predominating. [10]
Mechanism of activity
The mechanisms of biologic activity are poorly understood, and the predominant anatomic site of injury may vary, depending on the chemical species produced. Because of its intermediate water solubility and deeper penetration, elemental chlorine frequently causes acute damage throughout the respiratory tract. [11]
Cellular injury is believed to result from the oxidation of functional groups in cell components, from reactions with tissue water to form hypochlorous and hydrochloric acid, and from the generation of free oxygen radicals. Although chlorine was at one time thought to cause direct tissue damage by generating free oxygen radicals, [12] this concept is now considered controversial. [13, 14]
Solubility effects
While chlorine gas is only moderately soluble in water, hydrochloric acid is highly soluble. The predominant targets of the acid are the epithelia of the ocular conjunctivae and upper respiratory mucous membranes. [15]
Hypochlorous acid is also highly water soluble, with an injury pattern similar to hydrochloric acid. Hypochlorous acid may account for most of the toxic effects of chlorine to the human body. [16]
Physiologic response
The early response to chlorine exposure depends on the following [2] :
-
Concentration of chlorine gas
-
Duration of exposure
-
Water content of the tissues exposed
-
Individual susceptibility
The immediate effects of chlorine gas toxicity include acute inflammation of the conjunctivae, nose, pharynx, larynx, trachea, and bronchi. Irritation of the airway mucosa leads to local edema secondary to active arterial and capillary hyperemia. Plasma exudation into the alveoli results in pulmonary congestion and edema.
Pathologic findings
Pathologic findings are nonspecific. They include the following [17] :
-
Pulmonary edema
-
Pneumonia
-
Pneumonitis
-
Hyaline membrane formation
-
Multiple pulmonary thrombosis
-
Ulcerative tracheobronchitis
The hallmark of pulmonary injury associated with chlorine toxicity is pulmonary edema, manifested clinically as dyspnea, adventitious lung sounds, and hypoxia. Noncardiogenic pulmonary edema is thought to occur when there is a loss of pulmonary capillary integrity, and subsequent transudation of fluid into the alveolus. The onset can occur within minutes or hours, depending upon severity of exposure. Persistent hypoxemia is associated with a higher mortality rate.
In animal models of chlorine gas toxicity, immediate respiratory arrest occurs at 2000 ppm, with the lethal concentration for 50% of exposed animals in the range of 800-1000 ppm. [16] Bronchial constriction occurs in the 200-ppm range, with evidence of effects on ciliary activity at exposure levels as low as 18 ppm. With acute exposures of 50 ppm and subacute inhalation as low as 9 ppm, chemical pneumonitis and bronchiolitis obliterans have been noted. Mild focal irritation of the nose and trachea without lower respiratory effects occur at 2 ppm.
The extent of tissue response varies with both the concentration of exposure as well as underlying tissue sensitivity. In a study of chlorine gas toxicity conducted on human volunteers, 4 hours of exposure to chlorine at 1 ppm produced significant decreases in forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and peak expiratory flow rate, as well as an increase in airway resistance. [18]
Volunteers with hyperreactive airways were noted to experience an exaggerated airway response to exposure of 1 ppm chlorine gas. [19] In another study, patients with rhinitis and advanced age demonstrated a significantly greater nasal mucosal congestive response to chlorine gas challenge than did patients who did not have rhinitis or those of younger age. [20]
Eye injury
The eye is rarely damaged severely by chlorine gas toxicity; however, burns and corneal abrasions have occurred. Acids formed by the chlorine gas reaction with the conjunctival mucous membranes are partially buffered by the tear film and the proteins present in tears. Consequently, acid burns to the eye are typically limited to the epithelial and basement membrane, rarely extending to the deep endothelial cells.
Acid burns to the periphery of the cornea and conjunctiva often heal uneventfully. Burns to the center of the cornea may lead to corneal ulcer formation and subsequent scarring.
Ingestion
Ingestion is unlikely to occur, because chlorine is a gas at room temperature. Solutions that are able to generate chlorine (eg, sodium hypochlorite bleach) may cause corrosive injury if ingested. [8]
Etiology
Occupational exposures constitute the highest risk for serious exposure to high-concentration chlorine. Chlorine liquid is presently used in cleaning agents (eg, bleach, disinfectants), in water purification, and in the manufacture of items such as plastics. It is employed in the following industries:
-
Pesticide
-
Refrigerant
-
Paper and pulp
-
Textile
-
Metallurgy
-
Pharmaceutical
-
Cosmetic
-
Battery
-
Water and sewage purification
-
Food processing
Other exposures occur during industrial or transportation accidents. More than 200 significant industrial accidents involving chlorine have occurred since World War I. In addition, chemical attacks on civilians have been reported in both Iraq and, as stated above, Syria.
Household exposure occurs with chlorination tablet accidents during swimming pool maintenance, [21, 22, 23] or with inappropriate mixing of sodium hypochlorite (bleach) cleaning agents with ammonia products, which produces chloramine gas. Typically, this occurs in an enclosed environment such as a restroom. Chlorine gas also may be released in the household by mixing sodium hypochlorite solutions with acidic cleaning agents (toilet bowl cleaners).
Epidemiology
The 2023 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 41st Annual Report recorded 5079 single exposures to chlorine gas, with 14 major outcomes and no deaths. There were also 5329 single exposures to chlorine gas produced when household acid was mixed with hypochlorite, with six major outcomes and two deaths. In addition, the report recorded 3022 single exposures to chloramine gas, with seven major outcomes and no deaths. [24]
A retrospective study by Atalla et al suggested that in the United States, increased emphasis placed on disinfection practices during the coronavirus disease 2019 (COVID-19) pandemic may have contributed to a rise seen in exposures to chlorine and chloramine gas. Using information from the NPDS, the investigators reported that between 2015 and 2022, total exposures to chlorine and chloramine gas grew by 61%, with the period from 2019 to 2020 seeing the greatest increase, at 38.3%. The proportion of at-home exposures peri-pandemic was higher than that prepandemic, at 88.4% versus 81.7%, respectively. [25]
Prognosis
Most individuals exposed to chlorine gas recover without significant sequelae. Even exposure to high-concentration chlorine gas is unlikely to result in significant, prolonged pulmonary disease.
Morbidity from moderate and severe exposures is typically caused by noncardiogenic pulmonary edema. This may occur within 2-4 hours of exposure to moderate chlorine concentrations (25-50 ppm) and within 30-60 minutes of severe exposures (>50 ppm).
In serious exposures, sloughing of the pulmonary mucosa occurs in 3-5 days, and oozing areas become covered with mucopurulent exudate. This chemical pneumonitis is often complicated by secondary bacterial invasion.
Resolution of pulmonary abnormalities in most individuals occurs over the course of 1 week to 1 month after the exposure. Smokers and persons with asthma are most likely to demonstrate persistence of obstructive pulmonary defects. [12] In a study of patients exposed to chlorine gas released after a train derailment, hypoxia on room air and the ratio of partial pressure of oxygen to fractional concentration of oxygen in inspired air (PO2/FiO2) predicted the duration of hospitalization and the need for intensive care support. [26]
Residual effects
Although no definite conclusion can be drawn concerning the long-term effects of an acute chlorine gas exposure, findings suggest increased risk of persistent, nonspecific airway responsiveness. Furthermore, following an acute exposure, some patients with injured pulmonary epithelium have progressed to develop pulmonary fibrosis. [27] Bronchiolitis obliterans and emphysema have also been described in patients following acute exposures.
Significant immediate reductions in lung function were reported in a study of 1807 millworkers exposed to chlorine gas after a train derailment led to an estimated 54,915-kg release of chlorine in Graniteville, South Carolina. Improvement was seen in the second year; but the proportion of mill workers experiencing accelerated annual decline in FEV1 significantly increased in the 18 months following the exposure. In addition, the study found that smokers experienced additional FEV1 and FVC loss in the years after the accident. [28]
Irritant-induced asthma (formerly known as reactive airway dysfunction syndrome [RADS]) is a variant of occupational asthma that occurs after a single high-dose exposure or after repeated low-level exposures. [29] Within minutes to hours, those who have been exposed develop respiratory symptoms [30] followed by persistent bronchial hyperresponsiveness. [31]
Persistent anxiety after acute exposure to chlorine gas has been observed. In one large-scale accident, 37% of respondents had a positive posttraumatic stress screen 8-10 months post disaster, 44% of which were considered severe; 27% of all individuals had a positive indication for tendency to panic. Tendency to panic was significantly associated with acute injury and female sex. [32]
-
Chest radiograph of a 36-year-old chemical worker 2 hours postexposure to chlorine inhalant. She had severe resting dyspnea during the second hour, diffuse crackles/rhonchi on auscultation, and a partial pressure of oxygen of 32 mm Hg breathing room air. The radiograph shows diffuse pulmonary edema without significant cardiomegaly. Used with permission from Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. 1997: 256.
-
A section from a lung biopsy (hematoxylin and eosin stain; original magnification X 100) from a 36-year-old chemical worker taken 6 weeks postexposure to chlorine. At that time, the patient had no clinical abnormalities and a partial pressure of oxygen of 80 mm Hg breathing room air. The section shows normal lung tissues without evidence of interstitial fibrosis and/or inflammation. Used with permission from Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. 1997: 256.

