Practice Essentials
Although the terms decubitus ulcer, pressure sore, and pressure ulcer have often been used interchangeably, the National Pressure Injury Advisory Panel (NPIAP; formerly the National Pressure Ulcer Advisory Panel [NPUAP]) has stated that pressure injury the best term to use, given that open ulceration does not always occur. [1] According to the NPIAP, a pressure injury is localized damage to the skin and underlying soft tissue, usually over a bony prominence or related to a medical or other device. It can present as intact skin or an open ulcer and may be painful. It occurs as a result of intense or prolonged pressure or pressure in combination with shear.
See the image below.
Signs and symptoms
The following important information should be obtained from the history:
-
Overall physical and mental health, including life expectancy
-
Previous hospitalizations, operations, or ulcerations
-
Diet and recent weight changes
-
Bowel habits and continence status
-
Presence of spasticity or flexion contractures
-
Medications and allergies to medications
-
Tobacco, alcohol, and recreational drug use
-
Place of residence and the support surface used in bed or while sitting
-
level of independence, mobility, and ability to comprehend and cooperate with care
-
Underlying social and financial support structure
-
Presence of specific cultural, religious, or ethnic issues
Background
The terms decubitus ulcer (from Latin decumbere, “to lie down”), pressure sore, and pressure ulcer have often been used interchangeably in the medical community. However, as the name suggests, decubitus ulcer occurs at sites overlying bony structures that are prominent when a person is recumbent. Hence, it is not an accurate term for ulcers occurring in other positions, such as prolonged sitting (eg, ischial tuberosity ulcer). Because the common denominator of all such ulcerations is pressure, pressure ulcer came to be considered the best term to use.
The National Pressure Ulcer Advisory Panel (NPUAP) was an independent nonprofit organization formed in 1987 and dedicated to the prevention, management, treatment, and research of pressure ulcers. In April 2016, the NPUAP announced that it was changing its preferred terminology from pressure ulcer to pressure injury, on the grounds that the latter term better described this injury process in both intact and ulcerated skin. [1] In November 2019, the NPUAP changed its name to the National Pressure Injury Advisory Panel (NPIAP).
Currently, the NPIAP defines a pressure injury as localized damage to the skin and underlying soft tissue, usually over a bony prominence or related to a medical or other device. [1] Such injury can present either as intact skin or an open ulcer and may be painful. It results from intense or prolonged pressure or pressure combined with shear. The NPIAP also notes that the tolerance of soft tissue for pressure and shear may be affected by microclimate, nutrition, perfusion, comorbid conditions, and the condition of the soft tissue.
Pressure is exerted on the skin, soft tissue, muscle, and bone by the weight of an individual against a surface beneath. These pressures often exceed capillary filling pressure (~32 mm Hg). In patients with normal sensitivity, mobility, and mental faculty, pressure injuries do not occur. Feedback, conscious and unconscious, from the areas of compression leads them to change their body position, and these changes shift the pressure before any irreversible tissue damage develops. (See Pathophysiology and Etiology.)
Those who cannot avoid long-term uninterrupted pressure over bony prominences (eg, persons who are elderly, have neurologic impairment, or are undergoing acute hospitalization) are at increased risk for pressure injuries. They cannot protect themselves from the pressure unless they consciously change position or are helped to do so. Even a highly conscientious patient with an extensive support group and unlimited financial resources may develop such injuries as a result of a brief lapse in avoidance of the ill effects of pressure. [3, 4]
Addressing the overall management of pressure injuries is now a prominent national healthcare issue. Despite current interest and advances in medicine, surgery, nursing care, and self-care education, pressure injuries remain a major cause of morbidity and mortality, and patients with pressure injuries are important users of medical resources. [5, 6]
Many factors are involved in the management of pressure injuries. Nursing plays a pivotal role in this challenging and complex process, using a multifaceted approach that includes skin care, pressure relief, and nutritional support. Prevention is the key to managing pressure injuries, and it begins with a complete medical and nursing history, a risk assessment, and skin examination when the patient is admitted. (See Treatment.)
Factors that subject the tissue at risk to potential skin breakdown should receive particular attention. Patients should be kept clean and dry and should be repositioned frequently. For patients at risk, adequate pressure relief must be provided, along with adequate nutritional support.
For patients who develop pressure injuries, these preventive measures must be used in conjunction with the techniques of general wound care. Nonoperative wound care may involve simple topical therapy, as for pressure injuries with unbroken skin or superficial lesions with nondraining, noninfected granulation tissue. For draining necrotic or infected lesions, treatment also may include absorption agents, calcium alginate dressings, wound coverings, debridement, and antimicrobial therapy.
Other therapeutic modalities, such as whirlpool, physical therapy, and specialty beds, may also be added to the treatment regimen.
Research in the area of pressure injuries—specifically, in the characterization, prevention, and treatment of these lesions—is important for preventing secondary complications in persons with disabilities. As the standards of acute, posttraumatic, and rehabilitation care improve, the population of persons with lifelong functional impairments continues to grow. Consequently, the prevention of secondary complications has become an increasingly prominent concern.
To date, clinical studies of pressure injuries have been difficult to assess because they have often been qualitatively based on random observation and uncontrolled studies. To arrive at more reliable conclusions, more fundamental approaches to these injuries must be considered. Questions that might be asked include the following:
-
What are the basic histologic, pathologic, and biochemical markers in an evolving pressure injury?
-
Is it ethical to take a biopsy specimen of a human pressure injury for purposes of research?
-
What are the multiple variables in the formation of pressure injuries in the human environment?
A monograph prepared by the Research Committee of the NPUAP (now the NPIAP) suggested the following research priorities [7] :
-
Outcome-focused research
-
Intervention and product efficacy studies
-
Basic research related to staging of ulcers
-
Refinement of risk assessment methods
-
Risk-based, multi-interventional trials
Additional issues requiring investigation included cost issues, ethical decision making, guideline dissemination, public policy, and national outcome evaluations. Methodologic issues, such as research design, study population, and control group use, also were considered to warrant further investigation.
Anatomy
Pressure injuries are typically described in terms of location and depth of involvement. The hip and buttock regions account for up to 70% of all pressure injuries, with ischial tuberosity, trochanteric, and sacral locations being most common. [8] The lower extremities account for an additional 15-25% of all pressure injuries, with malleolar, heel, patellar, and pretibial locations being most common (see the images below).
The remaining small percentage of pressure injuries may occur in any location that experiences long periods of uninterrupted pressure. [8] The nose, chin, forehead, occiput, chest, back, and elbow are among the more common of the infrequent sites for pressure injuries. No surface of the body can be considered immune to the effects of pressure.
Pressure injuriescan involve different levels of tissue. Muscle has been proved to be most susceptible to pressure. However, Daniel and Faibisoff found that muscle rarely was interposed between bone and skin in normal weightbearing positions in cadaver and clinical dissections. [9]
Pathophysiology
In 1873, Sir James Paget described the production of pressure ulcers remarkably well, and his description is still quite accurate today. Many factors contribute to the development of pressure injuries, but pressure leading to ischemia and necrosis is the final common pathway.
In this view, pressure injuries result from constant pressure sufficient to impair local blood flow to soft tissue for an extended period. This external pressure must be greater than the arterial capillary pressure (32 mm Hg) to impair inflow and greater than the venous capillary closing pressure (8-12 mm Hg) to impede the return of flow for an extended time.
Tissues are capable withstanding enormous pressures for brief periods, but prolonged exposure to pressures just slightly above capillary filling pressure initiates a downward spiral toward tissue necrosis and ulceration. [10, 11] The inciting event is compression of the tissues against an external object such as a mattress, wheelchair pad, bed rail, or other surface.
Lindan et al documented ranges of pressure applied to various anatomic points in certain positions. [12] The points of highest pressure with the patient supine included the sacrum, heel, and occiput (40-60 mm Hg). With the patient prone, the chest and knees absorbed the highest pressure (50 mm Hg). When the patient is sitting, the ischial tuberosities were under the most pressure (100 mm Hg). Obviously, these pressures are greater than the end capillary pressure, which is why these are the areas where pressure injuries are most common.
Shear forces and friction aggravate the effects of pressure and are important components of the mechanism of injury (see the image below). Maceration may occur in a patient who has incontinence, predisposing the skin to injury. Pressure, shear forces, and friction cause microcirculatory occlusion and consequent ischemia, which leads to inflammation and tissue anoxia. Tissue anoxia leads to cell death, necrosis, and ulceration.
Of the various tissues at risk for death due to pressure, muscle tissue is damaged first, before skin and subcutaneous tissue, probably because of its increased need for oxygen and higher metabolic requirements. Irreversible changes may occur during as little as 2 hours of uninterrupted pressure. Skin can withstand ischemia from direct pressure for up to 12 hours. By the time ulceration is present through the skin level, significant damage of underlying muscle may already have occurred, making the overall shape of the ulcer an inverted cone.
Reperfusion has been suggested as a cause of additional damage to the ulcerated area, inducing an ulcer to enlarge or become more chronic—as, for example, when a paraplegic or quadriplegic patient is turned from one side to the other in an attempt to combat prolonged pressure on a given side. The exact mechanism of ischemia-reperfusion injury is yet to be fully understood. Continued production of inflammatory mediators and reactive oxygen species during ischemia-reperfusion may contribute to the chronicity of pressure ulcers.
Etiology
Impaired mobility is probably the most common reason why patients are exposed to the prolonged uninterrupted pressure that causes pressure injuries. This situation may be present in patients who are neurologically impaired, heavily sedated or anesthetized, restrained, demented, or recovering from a traumatic injury. These patients cannot alter their position far enough or often enough to relieve the pressure. Prolonged immobility may lead to muscle and soft tissue atrophy, decreasing the bulk over which bony prominences are supported.
Contractures and spasticity often contribute to ulcer formation by repeatedly exposing tissues to trauma through flexion of a joint. Contractures rigidly hold a joint in flexion, whereas spasticity subjects tissues to repeated friction and shear forces. Skin breakdown and pressure injuries may frequently be found under and between toes and on the palm of the hand.
Inability to perceive pain, whether from neurologic impairment or from medication, contributes to pressure injuries by removing one of the most important stimuli for repositioning and pressure relief. Conversely, pain from surgical incisions, fracture sites, or other sources may make the patient unwilling or unable to change position.
The quality of the skin also influences whether pressure leads to ulceration. Paralysis, insensibility, and aging lead to atrophy of the skin with thinning of this protective barrier. A decrease in epidermal turnover, a flattening of the dermal-epidermal junction, and a loss of vascularity occur with advanced age.
In addition, the skin becomes more susceptible to minor traumatic forces, such as the friction and shear forces typically exerted during the moving of a patient. Trauma that causes deepithelialization or skin tears removes the barrier to bacterial contamination and leads to transdermal water loss, creating maceration and causing the skin to adhere to clothing and bedding.
Incontinence or the presence of a fistula contributes to ulceration in several ways. These conditions cause the skin to be continually moist, thus leading to maceration. In addition, frequent soiling has the effect of regularly introducing bacteria into an open wound.
Bacterial contamination, though not truly an etiologic factor, must be considered in the treatment of pressure injuries, in that it can delay or prevent wound healing. These lesions are warm, moist reservoirs for bacterial overgrowth, where antibiotic resistance may develop. A pressure injury may progress from simple contamination (as in any open wound) to gross infection (indicating bacterial tissue invasion). This may lead to uncommon but life-threatening complications (eg, bacteremia, sepsis, myonecrosis, gangrene, or necrotizing fasciitis).
Malnutrition, hypoproteinemia, and anemia reflect the overall status of the patient and can contribute to tissue vulnerability to trauma as well as cause delayed wound healing. Poor nutritional status certainly contributes to the chronicity often seen in these lesions and inhibits the ability of the immune system to prevent infections. Anemia indicates poor oxygen-carrying capacity of the blood. Vascular disease and hypovolemia also may impair blood flow to the region of ulceration.
In patients with normal sensitivity, mobility, and mental faculty, pressure injuries are unlikely. Conscious or unconscious feedback from the areas of compression leads them to change position, thereby shifting the pressure from one area to another long before any irreversible ischemic damage occurs. In individuals who cannot avoid long periods of uninterrupted pressure, the risk of necrosis and ulceration is increased. These individuals cannot protect themselves from the pressure unless they consciously change position or are helped to do so.
Epidemiology
United States statistics
Pressure injuries are common among patients hospitalized in acute- and chronic-care facilities. It has been estimated that about 1 million pressure injuries occur in the United States; however, definitive information on the epidemiology and natural history of this condition is still limited. Unfortunately, studies to date have been encumbered by methodologic issues and variability in describing the lesions. [7]
Reported incidences of pressure injuries in hospitalized patients range from 2.7% to 29%, and reported prevalences in hospitalized patients range from 3.5% to 69%. [13, 14, 15, 16, 17] Patients in critical care units have an increased risk of pressure injuries, as evidenced by a 33% incidence and a 41% prevalence. [18, 19]
The fifth National Pressure Ulcer Prevalence Survey, conducted in 1999 among patients in acute care hospitals, showed an overall prevalence of 14.8%, with 7.1% of ulcers having occurred during that hospital visit. [20] Of the various hospital settings, intensive care units (ICUs) had the highest prevalence, at 21.5%. The largest single age group of patients with pressure injuries consisted of patients aged 71-80 years (29%).
Elderly patients admitted to acute care hospitals for nonelective orthopedic procedures are at even greater risk for pressure injuries than other hospitalized patients are, with a 66% incidence. [21, 22] In a study of 658 patients aged 65 years or older who underwent surgery for hip fracture, Baumgarten et al found that 36.1% developed an acquired pressure injury within 32 days after hospital admission. [23]
In nursing homes, the prevalence of pressure injuries is 2.6-24% [24] ; the incidence is 25% in residents admitted from an acute care hospital. [24] Patients with preexisting pressure injuries show a 26% incidence of additional pressure injury formation over a 6-month period. The incidence in chronic care hospitals is reported to be 10.8%, [25] whereas 33% of those admitted to a chronic care hospital have pressure injuries. [26] Long-term follow-up demonstrates that most ulcers heal within 1 year. [27]
Among patients with neurologic impairments, pressure injuries occur with an incidence of 7-8% annually, [28] with a lifetime risk estimated to be 25-85%. [29] Moreover, pressure injuries are listed as the direct cause of death in 7-8% of all individuals with paraplegia; these individuals also have the highest recurrence rate (80%). [30] In persons with spinal cord injury (SCI) and associated comorbidity, the incidence of pressure injuries is in the range of 25-66%. [31, 32, 33, 34]
A study of the prevalence of pressure injuries in community residents with SCI demonstrated that those with higher-level SCI lesions carry a greater risk of developing pressure injuries than those with lower-level lesions do. [31] Of 100 patients with pressure injuries, 33 had injuries that were classified as stage 2 or greater. Black patients had more severe injuries than other racial groups did.
Some authors have speculated that detecting erythema can be more difficult with skin that has darker pigmentation. [35] Because prolonged nonblanching erythema is typically an early warning sign of pressure injury risk and development, difficulty in detecting erythema can result in failure to recognize grade I pressure injuries.
International statistics
In a study from Germany that reviewed the prevalence of pressure injuries in more than 18,000 patients residing in long-term care facilities, the prevalence was found to have decreased from 12.5% in 2002 to 5% in 2008. [36] This decrease is thought to be due to more effective management strategies and better prevention.
Age-related demographics
The prevalence of pressure injuries appears to have a bimodal age distribution. A small peak occurs during the third decade of life, reflecting ulceration in those with traumatic neurologic injury. Immobility and lack of sensation make these patients susceptible to developing pressure injuries. Treatment of these lesions in this patient population represents a financial challenge, with one hospital reporting an average cost of $78,000 for each admission of a patient with a pressure injury.
As patients move from the age category of 40-58 years to the age category of 75 years or older, a larger increase in the incidence of pressure injuries occurs. [37] Two thirds of pressure injuries occur in patients older than 70 years. [25] As elderly individuals become the fastest-growing segment of the population, with an estimated 1.5 million people living in extended-care facilities, the problem of pressure injuries will have an even more profound influence on the American economy.
Sex-related demographics
Most younger individuals suffering from pressure injuries are males. The higher incidence in males reflects the greater number of men suffering traumatic SCIs. In the older population, most patients with pressure injuries are women, as a consequence of their survival advantage over men.
Race-related demographics
A study by Howard and Taylor found the incidence of pressure injuries in nursing home residents in the southeastern United States to be higher in Black patients than in White ones. [38] The authors examined data from 113,869 nursing home residents, none of whom had pressure injuries at nursing home admission. They determined that 4.7% of Black residents developed postadmission ulcerations, compared with 3.4% of White residents.
In addition, the racial differences in pressure injury incidence displayed a sex predilection based on patient characteristics. [38] The variation in incidence between Black and White males was noted in residents who were dependent in mobility, whereas the difference in incidence between Black and White females was noted in residents who were bedfast and living in nursing homes with fewer than 200 beds.
Prognosis
Pressure injuries are listed as the direct cause of death in 7-8% of all patients with paraplegia. [39, 28] As many as one third of hospitalized patients with pressure injuries die during their hospitalization. More than half of those who develop a pressure injury in the hospital will die within the next 12 months. As a rule, these patients die of their primary disease process rather than of pressure ulceration, but the pressure injury may be a contributing factor in some instances.
Each year, approximately 60,000 people die of complications of pressure injuries. [40] Individuals with pressure ulcers have a 4.5 times greater risk of death than persons with the same risk factors but without pressure injuries. A secondary complication, wound-related bacteremia, can increase the risk of mortality to 55%. [40, 41, 42, 43]
The most common causes of fatality for patients with chronic pressure injuries are renal failure and amyloidosis. In general, mortality is higher for patients who develop a new pressure injury and in whom the injury fails to heal.
Infection is the most common major complication of pressure injuries. The offending pathologic organisms can be either anaerobic or aerobic. Aerobic pathogens commonly are present in all pressure injuries, whereas anaerobes tend to be present more often in larger wounds (65% in grade 3 and above). [44]
The organisms most commonly isolated from pressure injuries are as follows:
-
Proteus mirabilis
-
Group D streptococci
-
Escherichia coli
-
Staphylococcus
-
Pseudomonas
-
Corynebacterium
Patients with bacteremia are more likely to have Bacteroides species in their pressure injuries. [44] These wounds need not be cultured routinely unless systemic signs of infection are present (eg, malodorous drainage, leukocytosis, fever, hypotension, increased heart rate, changes in mental status).
Clinical alertness is vital because the signs commonly associated with impeding or fulminating infection are frequently absent in elderly or immunocompromised patients. In geriatric patients with pressure injuries, bacteremia is reported to occur at a rate of 3.5 per 10,000 hospital discharges.
In view of the high mortality in this population (nearly 50%), [42] it is important that antibiotic treatment of wound infection or secondary bacteremia provide the appropriate spectrum of coverage specific to the offending organisms. Because indiscriminate use of antibiotics leads to resistant organisms and because the specific drugs of choice and antimicrobial agents change rapidly, management of these complex problems may be facilitated by consulting an infectious disease specialist.
Sepsis also can occur secondary to osteomyelitis, which has been reported to occur in 26% of nonhealing ulcers. A prospective study demonstrated that osteomyelitis was associated with nonhealing grade 4 pressure injuries in 86% of the study population. [45] This study utilized three-phase technetium methyl diphosphate radionuclide flow to detect early osteomyelitis.
Various tests can be used to diagnose osteomyelitis in patients with pressure injuries. Plain radiographs have a sensitivity of 78% and a specificity of 50%, but radiographic findings often are not present in the early stages of infection. Bone scans are more sensitive, but their specificity is low (50%). Bone biopsy has the highest specificity (96%) and sensitivity (73%). [45]
A combination of diagnostic tests (eg, white blood cell [WBC] count, erythrocyte sedimentation rate [ESR], and plain radiography) provides a sensitivity of 89% and a specificity of 88%. If all three test results are positive, the positive predictive value of this combination is 69%. If all three test results are negative, the negative predictive value is 96%. [45]
Osteomyelitis should be considered whenever an ulcer does not heal, especially if the ulcer is over a bony prominence. Clinicians also should rule out other conditions associated with nonhealing ulcers, such as heterotopic calcification or ossification. Most findings indicate that antibiotic treatment for osteomyelitis should last 6-8 weeks. Surgery is needed for some cases of chronic osteomyelitis.
Systemic amyloidosis can result from chronic suppurative pressure injuries. Additional complications of pressure injuries include spreading cellulitis, a sinus tract abscess, septic arthritis, squamous cell carcinoma in the ulcer, a periurethral fistula, and heterotopic ossification. Because some of the secondary complications of pressure injuries can preclude wound healing, they should be aggressively prevented and treated. Complications may include infection, pain, depression, and even death.
Patient Education
Patients and their support system must realize that it is their responsibility to avoid recurrent and new ulceration and that this is a lifelong process. Education on the proper avoidance of pressure should begin in the hospital and continue into the home.
For patient education resources, see the Skin, Hair, and Nails Center and Diabetes Center, as well as Wound Care and Diabetic Foot Care.
-
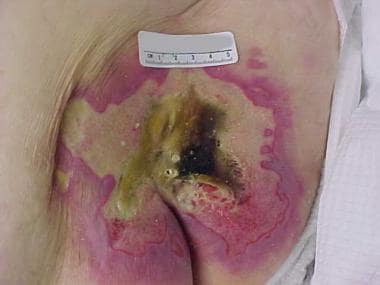
Advanced sacral pressure ulcer shows effects of pressure, shearing, and moisture.
-
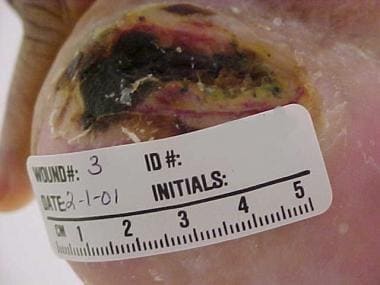
Heel pressure ulcer.
-
Small sacral pressure sores can be reconstructed with the inferior-based skin rotation flap, with or without the superior gluteus maximus myocutaneous flap.
-
Sacral pressure ulcer before and after flap closure.
-
Heaps of verrucous white tissue around the ulcer suggest malignant transformation, as observed with Marjolin ulcers.
-
Close-up view of area with heaps of verrucous white tissue around the ulcer, the presence of which suggests malignant transformation (as observed with Marjolin ulcers).
-
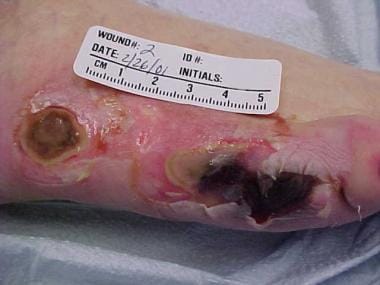
Pressure ulcers of lateral aspect of right foot.
-
Radical bursectomy is performed by placing methylene blue–moistened sponge in bursa and excising pressure ulcer circumferentially, removing all granulation tissue, even from wound base.
-
With gluteal thigh flap, superiorly based flap is elevated, with inferior gluteal artery located between greater trochanter and ischial tuberosity as its axis.
-
Gluteal thigh rotation flap is raised as fasciocutaneous flap superiorly to gluteal crease.
-
Gluteal thigh flap may be raised to include inferior portion of gluteus maximus, which increases arc of rotation to allow flap also to be used to reconstruct sacral defects.
-
Small sacral pressure ulcer reconstructed with inferiorly based skin rotation flap.
-
Small sacral pressure ulcer reconstructed with inferiorly based skin rotation flap.
-
Landmarks for superior gluteal artery, on which superior gluteus maximus muscle flap is based, include posterior superior iliac spine and ischial tuberosity.
-
Superior and inferior gluteal arteries branch from internal iliac superior and inferior arteries to piriformis approximately 5 cm from medial edge of origin of gluteus maximus from sacrococcygeal line.
-
When superior portion of gluteus maximus is used as flap, it is elevated in lateral-to-medial direction to avoid injury to superior gluteal artery. Insertion of superior portion of gluteus maximus into iliotibial tract is released. Harvesting entire length of muscle may be necessary to allow rotation or turnover into defect without tension.
-
V-Y flaps can be based superiorly or inferiorly or on entire gluteus maximus.
-
Larger sacral ulcers require use of bilateral flaps, such as bilateral V-Y advancement flaps.
-
Skin paddle is harvested 10 cm in width and designed over muscle along axis from anterior superior iliac spine to lateral tibial condyle.
-
Inferior limit of cutaneous territory can be extended to 6 cm above knee and 25-35 cm in length. Lateral femoral circumflex artery can be found approximately 6-8 cm inferior to anterior superior iliac spine.
-
Patient required reconstruction of extremely large pressure ulcer with fillet total thigh flap procedure.
-
Illustrated is Girdlestone arthroplasty for femoral head osteomyelitis pyarthrosis of hip joint. Femoral head is removed, and hip joint space is reconstructed with vastus lateralis muscle flap.
-
Patient has urethral fistula within his pressure ulcer. When he performs Valsalva maneuver, urine leaks through this opening.
-
Close-up view in patient who has urethral fistula within his pressure ulcer. When he performs Valsalva maneuver, urine leaks through this opening.
Tables
What would you like to print?
- Overview
- Presentation
- Workup
- Treatment
- Approach Considerations
- General Measures for Optimizing Medical Status
- Pressure Reduction
- Wound Management
- Principles of Surgical Intervention
- Surgical Debridement
- Options for Wound Closure
- Surgical Management of Specific Pressure Injury Types
- Postoperative Care
- Complications
- Activity
- Prevention
- Consultations
- Long-Term Monitoring
- Show All
- Guidelines
- Medication
- Media Gallery
- Tables
- References