Practice Essentials
Subglottic stenosis (SGS) is a narrowing of the subglottic airway, which is housed in the cricoid cartilage. The criterion standard for airway evaluation is direct laryngoscopy and direct bronchoscopy, while surgical therapies for subglottic stenosis (SGS) include serial endoscopic dilation, open reconstruction, anterior cricoid splitting, single-stage laryngotracheoplasty with cartilage expansion, anterior and posterior cricoid splitting with costal cartilage grafts placed anteriorly and/or posteriorly, and partial cricotracheal resection. The image below shows an intraoperative, endoscopic view of a normal subglottis. [1]
The subglottic airway is the narrowest area of the airway, since it is a complete, nonexpandable, and nonpliable ring, unlike the trachea, which has a posterior membranous section, and the larynx, which has a posterior muscular section. The term subglottic stenosis (SGS) implies a narrowing that is created or acquired, although the term is applied to both congenital lesions of the cricoid ring and acquired subglottic stenosis (SGS). See the images below.
 Grade III subglottic stenosis in a patient aged 18 years, following a motor vehicle accident. The true vocal cords are seen in the foreground. Subglottic stenosis is seen in the center of the picture.
Grade III subglottic stenosis in a patient aged 18 years, following a motor vehicle accident. The true vocal cords are seen in the foreground. Subglottic stenosis is seen in the center of the picture.
 Endoscopic view of the true vocal cords in the foreground and the elliptical congenital subglottic stenosis (SGS) in the center of the picture.
Endoscopic view of the true vocal cords in the foreground and the elliptical congenital subglottic stenosis (SGS) in the center of the picture.
 Subglottic view of very mild congenital subglottic stenosis. Laterally, the area looks only slightly narrow. When endotracheal tubes were used to determine its size, it was found to be 30% narrowed.
Subglottic view of very mild congenital subglottic stenosis. Laterally, the area looks only slightly narrow. When endotracheal tubes were used to determine its size, it was found to be 30% narrowed.
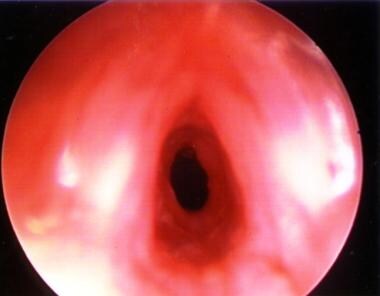 Granular subglottic stenosis in an infant aged 3 months who was born premature, weighing 800 g. The area is still granular following cricoid split. This patient required tracheotomy and eventual reconstruction at age 3 years. True vocal cords are shown in the foreground (slightly blurry).
Granular subglottic stenosis in an infant aged 3 months who was born premature, weighing 800 g. The area is still granular following cricoid split. This patient required tracheotomy and eventual reconstruction at age 3 years. True vocal cords are shown in the foreground (slightly blurry).
 Intraoperative laryngeal view of the true vocal cords of a boy aged 9 years. Under the vocal cords, a subglottic stenosis can be seen.
Intraoperative laryngeal view of the true vocal cords of a boy aged 9 years. Under the vocal cords, a subglottic stenosis can be seen.
 This spiraling subglottic stenosis is not complete circumferentially. Laser therapy was the treatment choice and was successful after 2 laser treatments.
This spiraling subglottic stenosis is not complete circumferentially. Laser therapy was the treatment choice and was successful after 2 laser treatments.
 Continued lasering of the subglottic stenosis. The reflected red light is the aiming beam from the CO2 laser.
Continued lasering of the subglottic stenosis. The reflected red light is the aiming beam from the CO2 laser.
 Postoperative view. Some mild residual posterior subglottic stenosis remains, but the child is asymptomatic and the airway is open overall.
Postoperative view. Some mild residual posterior subglottic stenosis remains, but the child is asymptomatic and the airway is open overall.
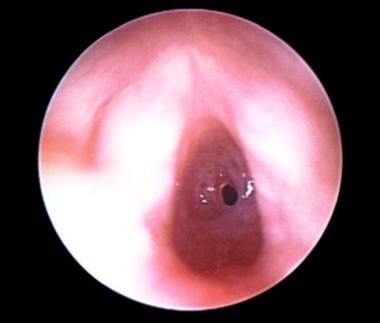 Preoperative view of an infant aged 4 months with acquired grade III subglottic stenosis from intubation. Vocal cords are in the foreground.
Preoperative view of an infant aged 4 months with acquired grade III subglottic stenosis from intubation. Vocal cords are in the foreground.
 A subglottic view following dilation with an endotracheal tube to lyse the thin web of scar and a short-course (5-day) treatment with oral steroids.
A subglottic view following dilation with an endotracheal tube to lyse the thin web of scar and a short-course (5-day) treatment with oral steroids.
 Postoperative view of an infant aged 4 months with subglottic stenosis, following cricoid split. Notice very mild recurrence of scarring at the site of a previous scar. Overall, the airway is open and patent. The anterior superior area can be seen, with a small area of fibrosis where the cricoid split previously healed.
Postoperative view of an infant aged 4 months with subglottic stenosis, following cricoid split. Notice very mild recurrence of scarring at the site of a previous scar. Overall, the airway is open and patent. The anterior superior area can be seen, with a small area of fibrosis where the cricoid split previously healed.
 Preoperative subglottic view of a patient aged 2 years with congenital and acquired vertical subglottic stenosis.
Preoperative subglottic view of a patient aged 2 years with congenital and acquired vertical subglottic stenosis.
Staging
Myer and Cotton devised a classification scheme for grading circumferential subglottic stenosis from I-IV, which is established endoscopically and by using noncuffed pediatric endotracheal tubes of various sizes and sizing the airway. The scale describes stenosis as a percent of area that is obstructed.
The system contains four grades, as follows:
-
Grade I - Obstruction of 0-50% of the lumen obstruction
-
Grade II - Obstruction of 51-70% of the lumen
-
Grade III - Obstruction of 71-99% of the lumen
-
Grade IV - Obstruction of 100% of the lumen (ie, no detectable lumen)
The percentage of stenosis is evaluated by using endotracheal tubes of different sizes. The largest endotracheal tube that can be placed with an air leak of less than 20 cm of water pressure is recorded and evaluated against the Cotton-Myer scale.
 Granulation tissue (superior center portion of the picture) that occurred at the graft site of a laryngotracheal reconstruction performed with an anterior graft.
Granulation tissue (superior center portion of the picture) that occurred at the graft site of a laryngotracheal reconstruction performed with an anterior graft.
This grading system applies mainly to circumferential stenosis and does not apply to other types of subglottic stenosis (SGS) or combined stenoses, although it can be used to obtain a rough estimate. Typically, children with grade I, as shown in the image below, or mild grade II stenosis do not require surgical intervention. Children with these conditions may have intermittent airway symptoms, especially when infection or inflammation causes mucosal edema.
 Subglottic view of very mild congenital subglottic stenosis. Laterally, the area looks only slightly narrow. When endotracheal tubes were used to determine its size, it was found to be 30% narrowed.
Subglottic view of very mild congenital subglottic stenosis. Laterally, the area looks only slightly narrow. When endotracheal tubes were used to determine its size, it was found to be 30% narrowed.
Monnier and colleagues have suggested using glottic involvement and associated medical comorbidities to subdivide the classic Cotton-Myer grading system for subglottic stenosis. Data from Monnier et al's initial report of this stratification demonstrated delayed time to decannulation in children with glottic involvement and higher failure rates in those with associated comorbidities, when compared with children with isolated SGS of the same Cotton-Myer grade. In the revised system, each grade is subdivided a through d, based on whether the patient has isolated SGS, SGS with comorbidity, SGS with glottic involvement, or SGS with glottic involvement and comorbidity (eg, Ia, Ib, Ic, Id, respectively). [2]
Workup in subglottic stenosis
Diagnostic procedures include the following:
-
Flexible fiberoptic nasopharyngoscopy and laryngoscopy
-
Flexible endoscopy
-
Rigid laryngoscopy and bronchoscopy
Certain radiographic examinations can help in obtaining a diagnosis and determining the severity of the disease, and fluoroscopy is sometimes performed in children with symptoms of airway obstruction.
Management of subglottic stenosis
Surgical therapies include the following:
-
Serial endoscopic dilation with or without steroid injections - For mild or granular subglottic stenosis (SGS)
-
Open reconstruction of subglottic stenosis (SGS) - Often may be unnecessary for subglottic stenosis (SGS) classified as grades I and II on the Cotton-Myer scale (ie, as much as 70% obstruction of the subglottic airway)
-
Anterior cricoid split - Children who are likely to benefit from this procedure include the following: (1) patient weight of more than 1500 g, (2) failure to extubate in identified subglottic stenosis (SGS), (3) oxygen requirement of less than 30%, (4) no active respiratory infection, and (5) good pulmonary and cardiac function
-
Single-stage laryngotracheoplasty with cartilage expansion
-
Anterior and posterior grafting - Anterior and posterior cricoid splitting with costal cartilage grafts placed anteriorly and posteriorly; has been successful in expanding the lumen and allowing decannulation in cases of severe subglottic stenosis (grades III-IV)
-
Partial cricotracheal resection - The best candidates for this procedure are patients with severe subglottic stenosis (grades III-IV) without associated glottic pathologic conditions and with a margin of at least 4 mm in the healthy airway below the vocal folds and above the stenosis
History of the Procedure
Early in the 20th century, acquired subglottic stenosis (SGS) was usually related to trauma or infection from syphilis, tuberculosis, typhoid fever, or diphtheria. Also, children often had tracheotomies placed that caused laryngeal stenosis. In this era, attempted laryngeal dilation failed as a treatment for subglottic stenosis (SGS).
Acquired subglottic stenosis (SGS) occurred increasingly in the late 1960s through the 1970s, after McDonald and Stocks (in 1965) introduced long-term intubation as a treatment method for neonates in need of prolonged ventilation for airway support. The increased incidence of subglottic stenosis (SGS) focused new attention on the pediatric larynx, and airway reconstruction and expansion techniques were developed. [3, 4, 5]
Surgery without cartilage expansion
In 1971, Rethi and Rhan described a procedure for vertical division of the posterior lamina of the cricoid cartilage with Aboulker stent placement. A metal tracheotomy tube was attached to the Aboulker stent with wires, and the anterior cartilaginous incision was closed. In 1974, Evanston and Todd described success with a castellated incision of the anterior cricoid cartilage and upper trachea, which was sewn open, with a stent made of a rolled silicone sheet placed in it for 6 weeks. In 1980, Cotton and Seid described a procedure, in which tracheotomy is avoided, called the anterior cricoid split (ACS). The technique was designed for use in neonates (usually, those born prematurely) with anterior glottic stenosis or SGS who had airway distress after extubation. The cricoid ring was divided anteriorly, and a laryngofissure was created in an attempt to expand the airway without a tracheotomy. Holinger et al also described success with this procedure in 1987.
Surgery with cartilage-grafting reconstruction
In 1974, Fearon and Cotton described the successful use of cartilage grafts to enlarge the subglottic lumen in African green monkeys and in children with severe laryngotracheal stenosis. [6] All augmentation materials were evaluated, including thyroid cartilage, septal cartilage, auricular cartilage, costal cartilage, hyoid bone, and sternocleidomastoid myocutaneous flaps. After significant work, it appeared that costal cartilage grafts had the highest success rate.
In the 1980s, Cotton reported his experience with laryngeal expansion with cartilage grafting. His success rates depended on the degree of stenosis: More severe forms of stenosis required multiple surgical procedures. Cotton used the Aboulker stent.
In 1991, Seid et al described a form of single-stage laryngotracheal reconstruction in which cartilage was placed anteriorly to expand the subglottis and upper trachea to avoid a tracheotomy. [7]
In 1992, Cotton et al described a four-quadrant cricoid split, along with anterior and posterior grafting. [8] In 1993, Zalzal reported 90% decannulation with any degree of subglottic stenosis (SGS) with his first surgical procedure. [9] Zalzal customized the reconstruction on an individual basis, and most patients received Aboulker stents for stabilization.
Cricotracheal resection
Early reports of partial cricotracheal resection were made in the 1960s by Ogura and Powers in a series of adolescents and adults and later by Savary in 1978 for acquired subglottic stenosis in a patient aged 10 years. [10] In 1993, Monnier et al described partial cricoid resection with primary tracheal anastomosis for severe SGS in children and infants. [11] Then, in 1997, Stern and colleagues reported their experience with partial cricotracheal resection with primary anastomosis, finding a decannulation rate higher than 90% for primary and rescue resection. [12]
Problem
Subglottic stenosis (SGS) is narrowing of the subglottic lumen. Subglottic stenosis (SGS) can be acquired or congenital. Acquired subglottic stenosis (SGS) is caused by either infection or trauma, as seen in the images below. Congenital subglottic stenosis (SGS) has several abnormal shapes.
 Grade III subglottic stenosis in a patient aged 18 years, following a motor vehicle accident. The true vocal cords are seen in the foreground. Subglottic stenosis is seen in the center of the picture.
Grade III subglottic stenosis in a patient aged 18 years, following a motor vehicle accident. The true vocal cords are seen in the foreground. Subglottic stenosis is seen in the center of the picture.
 Granular subglottic stenosis in an infant aged 3 months who was born premature, weighing 800 g. The area is still granular following cricoid split. This patient required tracheotomy and eventual reconstruction at age 3 years. True vocal cords are shown in the foreground (slightly blurry).
Granular subglottic stenosis in an infant aged 3 months who was born premature, weighing 800 g. The area is still granular following cricoid split. This patient required tracheotomy and eventual reconstruction at age 3 years. True vocal cords are shown in the foreground (slightly blurry).
 Intraoperative laryngeal view of the true vocal cords of a boy aged 9 years. Under the vocal cords, a subglottic stenosis can be seen.
Intraoperative laryngeal view of the true vocal cords of a boy aged 9 years. Under the vocal cords, a subglottic stenosis can be seen.
 This spiraling subglottic stenosis is not complete circumferentially. Laser therapy was the treatment choice and was successful after 2 laser treatments.
This spiraling subglottic stenosis is not complete circumferentially. Laser therapy was the treatment choice and was successful after 2 laser treatments.
 Continued lasering of the subglottic stenosis. The reflected red light is the aiming beam from the CO2 laser.
Continued lasering of the subglottic stenosis. The reflected red light is the aiming beam from the CO2 laser.
 Postoperative view. Some mild residual posterior subglottic stenosis remains, but the child is asymptomatic and the airway is open overall.
Postoperative view. Some mild residual posterior subglottic stenosis remains, but the child is asymptomatic and the airway is open overall.
 Preoperative view of an infant aged 4 months with acquired grade III subglottic stenosis from intubation. Vocal cords are in the foreground.
Preoperative view of an infant aged 4 months with acquired grade III subglottic stenosis from intubation. Vocal cords are in the foreground.
 A subglottic view following dilation with an endotracheal tube to lyse the thin web of scar and a short-course (5-day) treatment with oral steroids.
A subglottic view following dilation with an endotracheal tube to lyse the thin web of scar and a short-course (5-day) treatment with oral steroids.
 Postoperative view of an infant aged 4 months with subglottic stenosis, following cricoid split. Notice very mild recurrence of scarring at the site of a previous scar. Overall, the airway is open and patent. The anterior superior area can be seen, with a small area of fibrosis where the cricoid split previously healed.
Postoperative view of an infant aged 4 months with subglottic stenosis, following cricoid split. Notice very mild recurrence of scarring at the site of a previous scar. Overall, the airway is open and patent. The anterior superior area can be seen, with a small area of fibrosis where the cricoid split previously healed.
 Preoperative subglottic view of a patient aged 2 years with congenital and acquired vertical subglottic stenosis.
Preoperative subglottic view of a patient aged 2 years with congenital and acquired vertical subglottic stenosis.
Holinger evaluated 29 pathological specimens obtained in children with congenital cricoid anomalies. Half of these children had an elliptical cricoid cartilage, as shown below, which Tucker first described in 1979.
Elliptical cricoid cartilage was the most commonly observed congenital abnormality. Other observed abnormalities included a flattened anterior shape, a thickened anterior cricoid, and a submucosal posterior laryngeal cleft.
Epidemiology
Frequency
The frequency of congenital subglottic stenosis (SGS) is unknown.
The incidence of acquired subglottic stenosis (SGS) has greatly decreased since the late 20th century. In the late 1960s, when endotracheal intubation and long-term ventilation for premature infants began, the incidence of acquired subglottic stenosis (SGS) was as high as 24% in patients requiring such care. In the 1970s and 1980s, estimates of the incidence of subglottic stenosis (SGS) were 1-8%.
In 1998, Choi and Zalzal reported that the incidence of subglottic stenosis (SGS) had remained constant at the Children's National Medical Center in Washington, DC; it was approximately 1-2% in children who had been treated in the neonatal intensive care unit (ICU). [13] Walner reported that, among 504 neonates who were admitted to the level III ICU at the University of Chicago in 1997, 281 were intubated for an average of 11 days, with no patients developing subglottic stenosis (SGS) over a 3-year period. Moreover, in a systematic review published in 2001, Walner et al reported a decreasing incidence of neonatal subglottic stenosis in the literature, with studies published after 1983 finding an incidence of less than 4% and studies after 1990 having an incidence of under 0.63%. [14] In 1996, a report from France described no incidence of subglottic stenosis (SGS) in the neonatal population who underwent intubation with very small endotracheal tubes (ie, 2.5-mm internal diameter) in attempts to prevent trauma to the airway.
Morbidity
Using the Kids’ Inpatient Database (KID), Arianpour et al found that in addition to gastroesophageal reflux (GER), comorbidities more likely to be diagnosed in inpatients aged 20 years or younger with acquired subglottic stenosis (SGS) include trisomy 21, asthma, and additional upper airway anomalies. However, the chance of prematurity and dehydration was indicated to be lower in pediatric acquired SGS. [15]
Etiology
The cause of congenital subglottic stenosis (SGS) is in utero malformation of the cricoid cartilage.
The etiology of acquired subglottic stenosis (SGS) is related to trauma of the subglottic mucosa. Injury can be caused by infection or mechanical trauma, usually from endotracheal intubation but also from blunt, penetrating, or other trauma. Historically, acquired subglottic stenosis (SGS) has been related to infections such as tuberculosis and diphtheria. [16] Since the late 20th century, the condition has typically been related to mechanical trauma.
Factors implicated in the development of subglottic stenosis (SGS) include the size of the endotracheal tube relative to the child's larynx, the duration of intubation, the motion of the tube, and repeated intubations. Additional factors that affect wound healing include systemic illness, malnutrition, anemia, and hypoxia. Local bacterial infection may play an important role in the development of subglottic stenosis (SGS). Gastroesophageal reflux (GER) may play an adjuvant role in the development of subglottic stenosis (SGS) because it causes the subglottis to be continually bathed in acid, which irritates and inflames the area and prevents it from healing correctly. A systemic or gastrointestinal allergy may cause the airway to be more reactive, creating a greater chance of developing stenosis. Eosinophilic esophagitis has also been implicated in the development and propagation of stenoses.
A retrospective study by Pinzas et al found that in children who underwent tracheostomy and concurrent direct laryngoscopy, subsequent to endotracheal intubation and prior to follow-up direct laryngoscopy, glottic or subglottic stenosis developed with significantly greater frequency when glottic or subglottic injury was present at the time of initial direct laryngoscopy. Specifically, stenosis occurred in 30% of patients with injury versus 12% of those who were injury-free. The risk of developing airway injury was itself 2.33 times higher in members of the cohort with congenital heart disease than in those without it. [17]
A study by Lambercy et al found that in patients with intubation-related laryngeal lesions, intubation lasting over a week was associated with the development of more severe lesions and stenosis, which subsequently correlated with a higher rate of intervention and more invasive procedures, to avoid tracheostomy. [18]
A study by Schweiger et al indicated that in children who have been intubated, undersedation increases the risk that subglottic stenosis will develop. The study, of children aged 30 days to 5 years who underwent endotracheal intubation, found that the percentage of COMFORT behavior (COMFORT-B) scale scores between 23 and 30 (indicating undersedation) was greater in children with subglottic stenosis than in children without the condition (15.8% vs 3.65%, respectively). [19]
A study by Pavlek et al found that among patients who had undergone repeated unplanned extubations (UEs) in a neonatal ICU, the likelihood of developing acquired subglottic stenosis was significantly greater in those who had had at least four UEs and in whom the airway had suffered documented trauma during an intubation attempt, there was a history of tracheitis, and a major chromosomal anomaly or syndrome had been diagnosed. The results also indicated, however, that the odds of developing acquired subglottic stenosis are decreased in neonatal ICU patients who weighed less than 1500 g during their first UE. [20]
Pathophysiology
Acquired subglottic stenosis (SGS) is often caused by endotracheal intubation. Mechanical trauma from an endotracheal tube, as it passes through or remains for long periods in the narrowed neonatal and subglottic airway, can lead to mucosal edema and hyperemia. These conditions then can progress to pressure necrosis of the mucosa. Such changes have been observed within a few hours of intubation and may progress to expose the perichondrium of the cricoid cartilage. Infection of the perichondrium can result in a subglottic scar. This series of events can be hastened if an oversized endotracheal tube is used.
Always check for an air leak after placing an endotracheal tube, because of the risk of necrosis of the mucosa, even in short surgical procedures. This practice is common among anesthesiologists. Usually, the pressure of the air leak should be less than 20 cm of water so that no additional pressure necrosis occurs in the mucosa of the subglottis.
A study by Morrison et al indicated that in idiopathic subglottic stenosis (SGS), scar fibroblast production is driven by interleukin 17A (IL-17A). In addition, according to the investigators, IL-17A promotes extracellular matrix development by synergizing with transforming growth factor β1 (TGF-β1). Moreover, by prompting chemokine and cytokine production via direct scar fibroblast stimulation, IL-17A encourages the recruitment and differentiation of myeloid cells. [21]
A study by Ghavimi et al highlighted the role of TGF-β by demonstrating an increased inflammatory and fibrotic response to TGF-β stimulation from fibroblasts isolated from regions of subglottic stenosis, as compared with matched controls isolated from healthy regions of the distal trachea. [22]
Presentation
History
Children with subglottic stenosis (SGS) have an airway obstruction that may manifest in several ways. In neonates, subglottic stenosis (SGS) may manifest as stridor and obstructive breathing after extubation that requires reintubation. At birth, intubation in most full-term neonates should be performed with a 3.5-mm pediatric endotracheal tube. If a smaller-than-appropriate endotracheal tube must be used, narrowing of the airway may be present, which could suggest subglottic stenosis (SGS).
The stridor in subglottic stenosis (SGS) is usually biphasic. Biphasic stridor can be associated with glottic, subglottic, and upper tracheal lesions. Inspiratory stridor is usually associated with supraglottic lesions; expiratory stridor is usually associated with tracheal, bronchial, or pulmonary lesions.
The level of airway obstruction varies depending on the type or degree of subglottic stenosis (SGS). In mild subglottic stenosis (SGS), only exercise-induced stridor or obstruction may be present. In severe subglottic stenosis (SGS), complete airway obstruction may be present and may require immediate surgical intervention.
Depending on the severity, subglottic stenosis (SGS) can cause patients to have decreased subglottic pressure and a hoarse or a weak voice. Hoarseness or vocal weakness also can be associated with glottic stenosis and vocal cord paresis or paralysis.
Always ask about a history of recurrent croup. [23] A child with an otherwise adequate but marginal airway can become symptomatic with the development of mucosal edema associated with a routine viral upper respiratory infection (URI). Children with these conditions may have subglottic narrowing, and an evaluation of the airway is appropriate.
Always assess the history of GER disease (GERD). If present, always evaluate GERD prior to surgical intervention. A child who eventually has a diagnosis of subglottic stenosis (SGS) often has a history of either laryngotracheal trauma or intubation and ventilation. Frequently, these patients were born prematurely, have bronchopulmonary dysplasia, and may require oxygen administration. The degree of pulmonary disease and the amount of oxygen the child requires may affect the ability to perform decannulation. Prior to surgical intervention, the child should not require a substantial oxygen supplementation.
Physical examination
The physical examination varies depending on the degree of subglottic stenosis (SGS) present. Auscultate the child's lung fields and neck to assess any symptoms of airway obstruction and to evaluate pulmonary function. Completely evaluate the head and neck, and identify associated facial abnormalities such as cleft palate, choanal atresia, retrognathia, and facial deformities. Evaluate the child's initial overall appearance, and ask the following questions:
-
Is the child comfortable?
-
Does the child have difficulty breathing?
-
Does the child have difficulty breathing when emotionally upset?
-
Does the child have any suprasternal, substernal, or intercostal retractions?
-
Does the child have any nasal flaring?
-
Is the voice normal? Is it weak? Is the voice breathy?
-
Does the child have stridor? If so, what is the nature of the stridor (ie, inspiratory, expiratory, or biphasic)?
-
What is the child's neurologic status?
-
Does the child have a tracheotomy? Can the patient occlude the tracheotomy and still breathe without laboring?
Indications
Surgical reconstruction is performed on the basis of the symptoms, regardless of SGS grade, although children with grade I and mild grade II subglottic stenosis (SGS) often do not require surgical intervention.
Surgical intervention may be avoided if periods of airway obstruction are rare and can be treated on an inpatient or outpatient basis with anti-inflammatory and vasoconstrictive agents, such as oral, intravenous, or inhaled steroids and inhaled epinephrine (racemic treatment). If children with these conditions continue to have intermittent or persistent stridor and airway obstructive symptoms when they are well, or if they frequently become ill, surgical intervention may be necessary.
Development of upper respiratory symptoms during routine infections can indicate whether a child with subglottic stenosis (SGS) requires surgical reconstruction. Viral infections of the upper respiratory tract can create swelling in any area of the respiratory epithelium from the tip of the nose to the lungs. If a child with subglottic stenosis (SGS) has a cold and/or bronchitis but no significant symptoms of stridor or upper airway obstruction, the airway may be large enough to tolerate stress, and reconstruction may not be needed. A history of recurrent croup suggests subglottic stenosis (SGS).
Occasionally, older children have exercised-induced airway obstruction. At evaluation, these children may have grade I or grade II subglottic stenosis (SGS). Expansion of the airway with cartilage augmentation may allow them to lead a healthy and active lifestyle.
Children with grade III or IV subglottic stenosis (SGS) need one or more of the forms of surgical treatment discussed in Surgical Therapy.
Although croup, bacterial infection, GERD, and bronchopulmonary dysplasia may occur or be involved in the development of subglottic stenosis (SGS), a history of prolonged endotracheal tube intubation is the most common factor seen in patients with subglottic stenosis (SGS) that requires surgical correction.
Relevant Anatomy
The subglottis is defined as the area of the larynx housed by the cricoid cartilage, that extends from 5 mm beneath the true vocal cords to the inferior aspect of the cricoid ring, depicted in the image below. Because of the proximity and close relationship of the subglottis to the glottic larynx, glottic stenosis often can be present with subglottic stenosis (SGS). When SGS is corrected surgically, good voice quality can be preserved by not violating the true vocal cords if they are uninvolved in the disease process.
When creating the entry incision into the airway in an isolated subglottic stenosis (SGS), divide the cricoid cartilage, upper two tracheal rings, and the inferior third to half of the laryngeal cartilage in the midline; avoid dividing the anterior commissure. However, if the disease dictates or if exposure for repair cannot be obtained without dividing the anterior commissure, carefully perform the procedure. Endoscopic guidance can help in preventing injury to the glottic larynx.
If a laryngofissure is required for glottic stenosis or to gain access to the posterior aspect of the stenosis for suturing of the posterior graft, care must be taken to identify the anterior commissure and correctly put it back into place. Once the laryngofissure is created in the midline, immediately suture the anterior aspect of the true vocal chords near the anterior commissure to the laryngeal cartilage with a 6-0 monofilament suture such as polydioxanone (PDS) or Monocryl. This procedure helps to prevent the anterior commissure from becoming blunted and helps to mark approximately where it should be once the laryngofissure is closed.
When dividing the posterior cricoid lumen, note that the esophagus is immediately adjacent and posterior to it. Take care to avoid injuring the esophagus when completely dividing the posterior cricoid lamina during cartilage augmentation.
The recurrent laryngeal nerves enter the larynx in the posterior lateral portion of the cricoid ring. When surgery is performed in the midline, the recurrent laryngeal nerves should be far enough away from an anterior division to prevent injury. Any surgical procedure in which the lateral cricoid is divided could jeopardize the laryngeal nerve and result in paresis or paralysis of the true vocal cords. In the cricotracheal resection procedure, no attempt is made to identify the recurrent laryngeal nerves because of dense scarring. This lack of identification has resulted in some reported cases of paresis of the true vocal cord.
Contraindications
No specific absolute contraindications to the laryngotracheal reconstruction procedure exist. However, if general anesthesia is absolutely contraindicated, surgical correction of subglottic stenosis (SGS) cannot be performed.
A relative contraindication to reconstruction of a narrow subglottis is present in children who have a tracheotomy and subglottic stenosis (SGS) but need a tracheotomy for other purposes (eg, access for suctioning secretions caused by chronic aspiration) or in those who have airway collapse or obstruction in the nasal cavity, nasopharynx, oral cavity, oropharynx, supraglottic larynx, or trachea that cannot be repaired. However, if severe or complete laryngeal obstruction exists and if the child might be able to vocalize if the airway were surgically corrected, reconstruction may be beneficial, despite the need to maintain the tracheotomy tube.
Severe GER is another relative contraindication. Once GER is successfully treated (medically or surgically) or resolves on its own, reconstruction can be considered. An additional relative contraindication to airway reconstruction is pulmonary or neurologic function that is inadequate to withstand tracheotomy decannulation.
Eosinophilic esophagitis has also been implicated in the failure of laryngotracheal reconstruction. Hartnick et al reported in 2002 on a girl aged 2 years with a grade II subglottic stenosis, who demonstrated significant pharyngeal and post-cricoid inflammation, esophageal eosinophilia, and a normal pH probe study. The stenosis recurred rapidly after single-stage cricotracheal resection and only stabilized following initiation of a steroid regimen—at which time her inflammation had improved endoscopically and esophageal biopsies were devoid of eosinophilia. [24]
In a review of children diagnosed with eosinophilic esophagitis, 33% of patients required an otolaryngologic procedure over a 5-year period, with 1.4% undergoing laryngotracheal reconstruction. Of those requiring airway reconstruction, there was a 20% failure rate. [25] Similarly, in a review of 100 children undergoing cricotracheal resection at Cincinnati Children’s Hospital Medical Center, diagnosis of eosinophilic esophagitis was associated with lower operative-specific success rates. [26]
Regardless of the cause of subglottic stenosis (SGS), it is usually best to delay reconstructive efforts in children who have reactive or granular airways, shown below, until the reactive nature of the patient's condition subsides.
-
Intraoperative, endoscopic view of a normal subglottis
-
Grade III subglottic stenosis in a patient aged 18 years, following a motor vehicle accident. The true vocal cords are seen in the foreground. Subglottic stenosis is seen in the center of the picture.
-
Endoscopic view of the true vocal cords in the foreground and the elliptical congenital subglottic stenosis (SGS) in the center of the picture.
-
Subglottic view of very mild congenital subglottic stenosis. Laterally, the area looks only slightly narrow. When endotracheal tubes were used to determine its size, it was found to be 30% narrowed.
-
Subglottic view of congenital elliptical subglottic stenosis.
-
Granular subglottic stenosis in an infant aged 3 months who was born premature, weighing 800 g. The area is still granular following cricoid split. This patient required tracheotomy and eventual reconstruction at age 3 years. True vocal cords are shown in the foreground (slightly blurry).
-
Intraoperative laryngeal view of the true vocal cords of a boy aged 9 years. Under the vocal cords, a subglottic stenosis can be seen.
-
This spiraling subglottic stenosis is not complete circumferentially. Laser therapy was the treatment choice and was successful after 2 laser treatments.
-
Continued lasering of the subglottic stenosis. The reflected red light is the aiming beam from the CO2 laser.
-
Postoperative view. Some mild residual posterior subglottic stenosis remains, but the child is asymptomatic and the airway is open overall.
-
An intraoperative view of a split cricoid in a patient with elliptical congenital subglottic stenosis. The open airway can be seen in the center of the picture. The wound extends to the inferior one third of the thyroid cartilage.
-
Preoperative view of an infant aged 4 months with acquired grade III subglottic stenosis from intubation. Vocal cords are in the foreground.
-
A close-up view.
-
Postoperative view. The patient had been intubated for 1 week and extubated for 1 week.
-
A subglottic view following dilation with an endotracheal tube to lyse the thin web of scar and a short-course (5-day) treatment with oral steroids.
-
Postoperative view of an infant aged 4 months with subglottic stenosis, following cricoid split. Notice very mild recurrence of scarring at the site of a previous scar. Overall, the airway is open and patent. The anterior superior area can be seen, with a small area of fibrosis where the cricoid split previously healed.
-
Rib graft for reconstruction of subglottic stenosis. The diamond-shaped internal intraluminal component with perichondrium still present is seen on the top section of the rib, and the shape of the rib is seen on the backside of the carved-out diamond shape.
-
Anterior rib graft with a diamond shape. Note it measures approximately 1.7 mm in length. Intraluminal site is facing up. Flanges of rib are carved to remain on the outside of the trachea to prevent prolapse into the trachea.
-
Cartilage graft in place over the wound. Note external component of the graft still looks like a portion of the rib. The internal component has been carved in a diamond shape. This is an intraoperative photo. The cartilage graft was used in this patient for reconstruction.
-
Graft with all sutures in position. All the sutures are placed prior to lowering the graft into position. Then, the sutures are tied.
-
Representative sample of varying sizes of Aboulker stents (range of 3-15 mm). These stents are hollow and coated in Teflon.
-
Endoscopic view of Aboulker stent protruding through and above the true vocal cords. The arytenoids and epiglottic folds are seen.
-
Diagram of a long Aboulker stent wired to a metal Jackson tracheotomy tube.
-
A Jackson tracheotomy tube wired to a long Aboulker stent.
-
A 7-mm Montgomery tracheotomy tube with caps
-
Granulation tissue (superior center portion of the picture) that occurred at the graft site of a laryngotracheal reconstruction performed with an anterior graft.
-
Intraoperative, suspended view with a subglottoscope of the subglottis, showing the granulation tissue just prior to removal with cup forceps and laser.
-
Postexcision view of granulation tissue through the subglottoscope.
-
Preoperative view of glottic stenosis and small glottic chink in a child aged 2 years.
-
Preoperative subglottic view of a patient aged 2 years with congenital and acquired vertical subglottic stenosis.
-
Postoperative view of the glottic larynx in a child who underwent anterior and posterior grafting for subglottic stenosis. Note the glottis is more open and in neutral position. The scarring of the right true vocal cord appears improved.
-
Postoperative, close-up view of the true vocal cords.
-
Postoperative, subglottic view of patient who underwent anterior and posterior grafting with successful decannulation, showing open subglottis with some very mild damage to the anterior wall and the suprastomal area where the tracheostomy tube had been placed
-
Subglottic view of an anterior graft, placed for anterior subglottic stenosis. The white areas to the left and right are the true vocal cords. The graft is seen at the superior and mid area.
-
Intraoperative view of an A-frame deformity of the trachea at the site of a prior tracheostomy.





