Practice Essentials
The term macrosomia is used to describe a newborn with an excessive birth weight. An accurate diagnosis of fetal macrosomia can be made only by measuring birth weight after delivery; therefore, the condition is confirmed only retrospectively, ie, after delivery of the neonate. Fetal macrosomia has been defined in several different ways, including birth weight greater than 4000 g or 4500 g (8 lb 13 oz or 9 lb 15 oz) or greater than 90% for gestational age. [1] According to National Vital Statistics Report for U.S. Births in 2017, approximately 7.8% of infants had birth weight >4000 g, 1% had birth weight greater than 4500 g, and 0.1% had birth weight greater than 5000 g. [2]
Attempts at perinatal diagnosis of macrosomia have proven difficult and are often inaccurate. This article defines macrosomia and reviews clinical and diagnostic modalities currently used to screen for pregnancies at the greatest risk for macrosomia with some degree of accuracy. Maternal, fetal, and neonatal consequences of macrosomia are also discussed, with specific attention to the potential etiology of macrosomia.
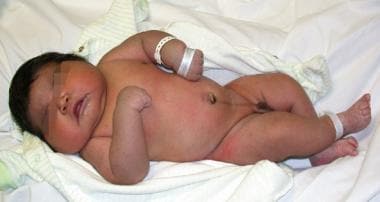
See the image below.
Background
Factors associated with fetal macrosomia include genetics; duration of gestation; presence of gestational diabetes; high pre-pregnancy body mass index (BMI); excessive gestational weight gain; and class A, B, and C diabetes mellitus. [3] Genetic, sex, racial, and ethnic factors influence birth weight and the risk of macrosomia. [4] Male newborns typically weigh more than female newborns and thus comprise a greater proportion of infants with birth weights exceeding 4500 g. The risk of macrosomia also varies with ethnicity. Even when controlled for diabetes, studies have demonstrated that Hispanic women have a higher risk of fetal macrosomia compared with White, African American, or Asian women. Genetic factors, such as parental height and weight, may also play a role in determining newborn birth weight.
Despite the identification and characterization of risk factors, no combination of these risk factors can predict macrosomia accurately enough to be used clinically. Much of the birth weight variation remains unexplained, and most macrosomic infants do not have identifiable risk factors. Finally, macrosomia is reportedly associated with neonatal morbidity, neonatal injury, maternal injury, and cesarean delivery. [5, 6] Risks associated with macrosomia increase on a continuum. Macrosomia is often divided into three categories with different levels of risk: (1) 4000-4499 g, (2) 4500-4999 g, and (3) more than 5000 g. [1]
Pathophysiology
The pathophysiology of macrosomia is related to the associated maternal or fetal condition that accounts for its development. In general, poorly controlled diabetes, maternal obesity, and excessive maternal weight gain are all associated with macrosomia and have intermittent periods of hyperglycemia in common. Hyperglycemia in the fetus results in the stimulation of insulin, insulin-like growth factors, growth hormone, and other growth factors, which, in turn, stimulate fetal growth and deposition of fat and glycogen. Advanced gestational age results in a larger birth weight at delivery by allowing the growth process to continue in utero.
Various studies have examined the in utero development of macrosomic fetuses. In a secondary analysis of data from a randomized control trial on treatment versus non-treatment of mild gestational diabetics, Stuebe et al assessed the link between maternal body mass index (BMI), glucose intolerance, and fetal and maternal risk factors. Stuebe et al found pre-gravid maternal BMI is linked with macrosomia and with increased neonatal fat mass independent of oral glucose challenge test values. [7] Another study performed by Catalano et al further elucidates this link in their analysis of >400 infants born to women with and without glucose intolerance. They found that infants born to women with glucose intolerance have increased fat mass when compared to infants born to women with normal glucose tolerance. This was independent of maternal BMI. [8] Geraghty et al collected blood samples on 331 mother-child pairs in a prospective cohort. They found that maternal serum triglycerides correlated positively with birth weight. [9] These studies highlight the complexity of the pathophysiology leading to macrosomia and also demonstrate that both maternal obesity and maternal glucose intolerance not only increase birth weight but also lead to increased neonatal adiposity or percent body fat, in turn putting them at increased risk of macrosomia and its complications, including shoulder dystocia, birth injury, neonatal intensive care unit admission, and even fetal death. [7, 8, 10, 11]
Macrosomia may be associated with birth trauma for the neonate and birth canal lacerations, eg, perineal, vaginal, and cervical, [12] or cesarean delivery for the mother. A large for gestational age fetus in a diabetic mother may indicate poor glucose control. These infants are at increased risk of intrauterine death [13] and thus require close monitoring and antepartum fetal testing.
Etiology
Causes for macrosomia include factors that contribute to excessive fetal growth and weight gain.
There are numerous contributors to macrosomia, many of which are assessed in a case-control study by Okun et al, which list factors including: prior macrosomic infant, maternal prepregnancy weight, excessive gestational weight gain, multiparity, male fetus, gestational age >40 weeks, ethnicity, maternal birth weight, maternal height, maternal age younger than 17 years, and a positive 50-g glucose screen with a normal 100-g glucose tolerance test, in descending order of effect according to their analysis of data from 1000 deliveries of macrosomic and non-macrosomic infants in Edmonton, Alberta. [4]
A study done by Kim et al reviewed vital records between 2004 and 2008 in Florida to assess the association between maternal BMI, maternal weight gain, and gestational diabetes mellitus with fetal macrosomia. They found that excessive maternal weight gain had the strongest association for a large for gestational age infant of the examined variables. BMI of greater than 25 and gestational diabetes were also associated with having a large-for-gestational-age (LGA) infant. Initial BMI and gestational weight gain are also both modifiable risk factors and provide potential interventions to decrease a patient’s risk of having a macrosomic infant. [3]
Poor glycemic control in pregnancy is a major risk factor for fetal macrosomia. Maternal glucose passes through the placenta, leading to fetal hyperglycemia and hyperinsulinemia as well as an increase in levels of insulin-like growth factors and growth hormone. This leads to increased fetal fat deposition, glycogen synthesis, and larger fetal size. Elevated fasting glucose levels may be more strongly associated with macrosomia. [14]
Genetic factors also contribute to fetal size. Taller (80th percentile or more) and heavier parents typically produce larger offspring. Women with short stature and obesity are at almost threefold higher risk for macrosomia compared with those of short stature with normal/overweight BMI. [15]
Epidemiology
United States data
Of US births in 2017, approximately 7.8% of infants had birth weight >4000 g, 1% had birth weight greater than 4500 g, and 0.1% had birth weight greater than 5000 g. [2] Rates of large-for-gestational-age (LGA) newborns are increased in women with gestational diabetes mellitus (GDM), with 13.6% of fetuses being macrosomic in normal-weight women and 22.3% in obese women. [16]
Race- and sex-related demographics
Macrosomia occurs with higher frequency in newborns of Hispanic origin. Part of the preponderance of macrosomia in this ethnic group may be due to the higher incidence of diabetes in pregnancy. However, even when corrected for diabetes, Hispanic mothers tend to have larger newborns.
Male infants are more likely to be macrosomic than female infants. Male infants are generally approximately 150-200 g larger than female infants of the same gestational age near term. [17, 18]
Gestational age
Macrosomia, as defined by birth weight greater than 4000-4500 g, occurs with higher frequency in prolonged pregnancies that continue beyond the expected delivery date. This is to be expected, as infants gain approximately 150-200 g weekly near term. [17]
Prognosis
There is some evidence that link may exist between macrosomia and longstanding health issues that develop later in life, such as insulin resistance, hypertension, and obesity. A study of macrosomic mice found that males with macrosomia had increased likelihood of heavier body weight, insulin resistance, and impaired glucose tolerance compared to non-macrosomic males. In females, there was a link with macrosomia and higher blood pressures, rather than body weight or glycemic control issues. [19]
A cross-sectional study of adults with type 2 diabetes in China found an association between macrosomia and increased abdominal obesity as well as increased rates of hypertension. [20] Furthermore, a meta-analysis performed by Harder et al, found a “U-shaped” relationship between birth weight and risk of type 2 diabetes mellitus later in life, meaning infants born at both extremes of birth weight, both SGA and LGA, are at increased risk of developing type 2 diabetes in adulthood. [21] However, a study by Callanan et al found no strong evidence that macrosomia alone is a risk factor for worse cardiometabolic health in pre-teenage children. [22]
Morbidity/mortality
Morbidity and mortality associated with macrosomia can be divided into maternal, fetal, and neonatal categories. A study investigating the effects of birth weight on fetal mortality shows that higher fetal mortality rates are associated with a birth weight of greater than 4250 g in nondiabetic mothers and a birth weight of 4000 g in diabetic mothers. [13]
Maternal morbidity
Macrosomia is associated with a higher incidence of cesarean delivery (double that of control subjects) and with birth canal lacerations associated with vaginal delivery. Labor protraction and arrest disorders are more common with fetal macrosomia. The preponderance of cesarean deliveries related to macrosomia are due to abnormal labor. [1] There is a twofold to threefold increased risk of third-degree and fourth-degree lacerations associated with macrosomia. [23] The risks of postpartum hemorrhage and chorioamnionitis are also increased. [24]
Mulik et al reviewed the outcomes of 8617 deliveries over a period of 11 years. [25] In that population, 666 neonates were born with a birth weight of 4000-4499 g and 97 neonates were larger than 4500 g. In their study, Mulik et al found maternal morbidity to be associated with a birth weight of 4500 g or higher compared with a birth weight of less than 4000 g. Postpartum hemorrhage occurred in 3.1% of mothers with newborns weighing 4500 g or more compared with 1.5% in mothers with newborns weighing less than 4000 g. Blood transfusions occurred in 15.4% of mothers with newborns weighing 4500 g or more compared with 3.1% in mothers with newborns weighing less than 4000 g.
Neonatal morbidity
Macrosomic neonates are at risk for shoulder dystocia and birth trauma. The common injuries are clavicular fracture and brachial plexus injury, specifically C5-C6 leading to Erb-Duchenne paralysis. [26] This risk is directly related to neonatal birth weight and begins to increase substantially when birth weight exceeds 4500 g and particularly when it exceeds 5000 g. Brachial plexus injury is rare, with an incidence of fewer than 2 cases per 1000 vaginal deliveries. This risk is approximately 20 times higher when the birth weight is more than 4500 g. [12] Risk of fracture of the clavicle is approximately 0.4-0.6% of all births, increasing 10-fold for macrosomic newborns. [26] Mulik et al reported a higher incidence of NICU admissions for neonates with a birth weight higher than 4500 g compared with newborns with a birth weight of less than 4000 g (9.3% vs 2.7%). Risk of shoulder dystocia was 10 times higher in the larger babies (4.1% vs 0.4%).
In a large study by Raio et al, 3356 newborns who weighed more than 4500 g at birth were studied. Shoulder dystocia occurred in 310 of the newborns, and brachial plexus injuries occurred in 94 of the newborns (about 10% and 3%, respectively). In this population, gestational diabetes increased the risk of shoulder dystocia by a factor of two, while preexisting diabetes increased the risk fourfold. [27]
Fetal morbidity/mortality
When associated with diabetes, fetal macrosomia indicates poor maternal glucose control, and these infants are at risk of stillbirth. Mondestin et al investigated the effects of birth weight on fetal mortality and demonstrated that higher fetal mortality rates are associated with a birth weight of greater than 4250 g in nondiabetic mothers and a birth weight of 4000 g in diabetic mothers. [13] Stillbirth rates in macrosomic infants are twice as high as those in control subjects, irrespective of diabetes. However, for a birth weight of 4500-5000 g, the fetal death rate is fewer than 2 deaths per 1000 births for nondiabetic women and is approximately 8 deaths per 1000 births for diabetic women. For a birth weight of 5000-5500 g, this rate is 5-18 deaths per 1000 births for nondiabetic women and is approximately 40 deaths per 1000 births for diabetic women. [13]
A retrospective cohort analysis designed to demonstrate the link between SGA and perinatal demise showed a “reverse J-shaped relationship” between birth weight percentile and risk of fetal and neonatal death. This means that the greatest risk of perinatal death is at birth weights ≤3rd percentile and ≥98th percentile. A great deal of research and management guidance has gone into surveillance for SGA infants, and this study would suggest that more investigation is warranted to examine the perinatal risks and optimal surveillance of the fetus with accelerated growth. [28]
This is supported by a study by Boulet et al that showed increased risk of neonatal death with increasing birth weight, most notable and statistically significant for infants with birth weights greater than 5000 g, or “grade 3 macrosomia” according to the model in their study. [6]
A large retrospective cohort study by Linder et al included singleton, full-term newborns with a birth weight of ≥4000 g at a tertiary care center, who were matched with healthy newborns with a birth weight of 3000-4000 g. The results showed that macrosomic infants had higher rates of hypoglycemia, transient tachypnea of the newborn, hyperthermia, and birth trauma. There were no differences between the two groups in low 5-minute Apgar scores, metabolic acidosis, infection, loss of >10% of body weight, meconium aspiration syndrome, or cyanotic episodes. Two additional studies that examined infants of diabetic mothers suggested that in utero exposure to hyperglycemia leads to disproportionate growth and higher rates of neonatal complications, such as hypoglycemia, hyperbilirubinemia, polycythemia, and metabolic acidosis. [29]
Patient Education
As with obesity, excessive maternal weight gain can be prevented by appropriate education of expecting mothers regarding weight gain in pregnancy. Such interventions may reduce the risk of macrosomia in specific pregnancies that may have been placed at risk because of excessive maternal weight gain. However, although excessive maternal weight or weight gain in pregnancy has been associated with fetal macrosomia, the effectiveness of reducing prepregnancy weight or curtailing excessive weight gain in pregnancy has not been tested to determine whether these measures will reduce rates of fetal macrosomia. Furthermore, one must consider the risk of insufficient gestational weight gain including increased risk of growth restriction. [30]
-
Photograph of a macrosomic newborn soon after birth.