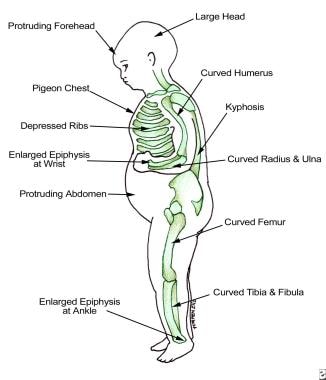
Practice Essentials
Vitamin D deficiency can result from inadequate exposure to sunlight, malabsorption, accelerated catabolism from certain medications, and, in infants, the minimal amount of vitamin D found in breast milk. In children, vitamin D deficiency can result in rickets, which presents as bowing of the legs; in adults, it results in osteomalacia, which presents as a poorly mineralized skeletal matrix. These adults can experience chronic muscle aches and pains. [1] (See the images below.)
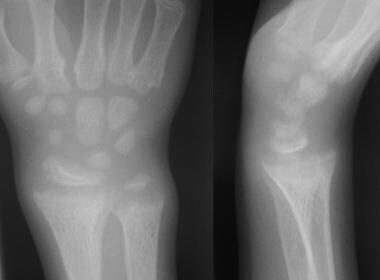 Anteroposterior and lateral radiographs of the wrist of an 8-year-old boy with rickets demonstrates cupping and fraying of the metaphyseal region.
Anteroposterior and lateral radiographs of the wrist of an 8-year-old boy with rickets demonstrates cupping and fraying of the metaphyseal region.
Signs and symptoms
Vitamin D deficiency is often clinically silent. Manifestations are as follows:
-
Children are often found to have started walking late or prefer to sit down for prolonged periods
-
Adults can experience chronic muscle aches and pains
Physical findings in severe vitamin D deficiency are as follows:
-
In children, bowing in the legs
-
In adults, periosteal bone pain, best detected with firm pressure on the sternum or tibia
See Clinical Presentation for more detail.
Diagnosis
Measurement of serum 25-hydroxyvitamin D (25[OH]D) is the best test to determine vitamin D status. Levels of 25(OH)D are interpreted as follows [2, 3] :
-
12-< 20 ng/mL: Vitamin D insufficiency
-
< 12 ng/mL: Vitamin D deficiency
Although not always required for the diagnosis of vitamin D insufficiency, measurement of the serum parathyroid hormone (PTH) level may help to establish the diagnosis of insufficiency. PTH levels are often elevated in patients with vitamin D insufficiency, indicating secondary hyperparathyroidism.
Screening for vitamin D deficiency is recommended only in those individuals who are at high risk for vitamin D deficiency, including the following [4] :
-
Patients with osteoporosis
-
Patients with a malabsorption syndrome
-
Black and Hispanic individuals
-
Persons with obesity (body mass index >30 kg/m2) [5]
-
Patients with disorders that affect the metabolism of vitamin D and phosphate (eg, chronic kidney disease)
Indeed, the Endocrine Society now recommends against screening for vitamin D deficiency in most healthy adults. [6]
See Workup for more detail.
Management
Recommended treatment for vitamin D–deficient patients up to age 1 year is as follows [4] :
-
2000 IU/day of vitamin D2 or D3 for 6 weeks or
-
50,000 IU of vitamin D2 or D3 once weekly for 6 weeks
-
When the serum 25(OH)D level exceeds 30 ng/mL, provide maintenance treatment of 400-1000 IU/day
Recommended treatment for vitamin D–deficient patients 1-18 years of age is as follows [4] :
-
2000 IU/day of vitamin D2 or D3 for at least 6 weeks or
-
50,000 IU of vitamin D2 once weekly for at least 6 weeks
-
When the serum 25(OH)D level exceeds 30 ng/mL, provide maintenance treatment of 600-1000 IU/day
Recommended treatment for vitamin D–deficient adults is as follows [4] :
-
50,000 IU of vitamin D2 or D3 once weekly for 8 weeks or
-
6000 IU/day of vitamin D2 or D3 for 8 weeks
-
When the serum 25(OH)D level exceeds 30 ng/mL, provide maintenance treatment of 1500-2000 IU/day
Recommended treatment for vitamin D–deficient patients who have obesity or a malabsorption syndrome or are taking medication that affects vitamin D metabolism is as follows [4] :
-
At least 6000-10,000 IU of vitamin D daily
-
When the serum 25(OH)D level exceeds 30 ng/mL, provide maintenance treatment of 3000-6000 IU/day
Endocrine Society guidelines (2024) suggest that adults aged 50 years or above in whom vitamin D supplementation or treatment is indicated receive "daily, lower-dose vitamin D instead of nondaily, higher-dose vitamin D." [6]
If the 25(OH)D concentration remains persistently low despite several attempts at correction with oral vitamin D, a trial of ultraviolet-B light therapy (ie, by tanning lamps) may be considered to improve vitamin D status.
Prevention
Unprotected sun exposure is the major source of vitamin D for both children and adults. [4] Provision of vitamin D from sunlight is as follows:
-
Sensible sun exposure, especially between the hours of 10 am and 3 pm, produces vitamin D in the skin that may last twice as long in the blood compared with ingested vitamin D [7]
-
Full-body sun exposure producing slight pinkness in light-skinned persons results in vitamin D production equivalent to ingesting 10,000-25,000 IU [8]
-
Increased skin pigmentation, aging, and sunscreen use reduce the skin’s vitamin D3 production
Recommended dietary intake of vitamin D for patients at risk of vitamin D deficiency is as follows [4] :
-
In infants and children up to age 1 year, at least 400 IU/day, to maximize bone health
-
In children and adolescents aged 1-18 years, at least 600 IU/day to maximize bone health
-
In adults aged 19-50 years, at least 600 IU/day to maximize bone health and muscle function
-
Raising the serum 25(OH)D level consistently above 30 ng/mL may require vitamin D intake of at least 1000 IU/day
-
Whether recommended levels of vitamin D intake will provide all the potential nonskeletal health benefits associated with vitamin D is currently unknown
Most dietary sources of vitamin D do not contain sufficient amounts of the vitamin to satisfy daily requirements. The following foods contain the indicated amounts of vitamin D, as reported by the US Department of Agriculture's (USDA's) Nutrient Data Laboratory:
-
Fortified milk (8 oz) - 100 IU
-
Fortified orange juice (8 oz) [9] - 100 IU
-
Fortified cereal (1 serving) - 40-80 IU
-
Pickled herring (100 g) - 680 IU
-
Canned salmon with bones (100 g) - 624 IU
-
Mackerel (100 g) - 360 IU
-
Canned sardines (100 g) - 272 IU
-
Codfish (100 g) - 44 IU
-
Swiss cheese (100 g) - 44 IU
-
Raw shiitake mushrooms (100 g) - 76 IU
-
Most multivitamins (1 tab) - 400 IU
See Treatment and Medication for more detail.
Background
Vitamin D is important for calcium homeostasis and for optimal skeletal health. The major function of vitamin D is to increase the efficiency of calcium absorption from the small intestine. Heaney and colleagues demonstrated that maximum calcium absorption occurs at levels of 25-hydroxyvitamin D (25[OH]D) greater than 32 ng/mL. [10] (See Pathophysiology and Etiology.)
Vitamin D also enhances the absorption of phosphorus from the distal small bowel. Adequate calcium and phosphorus absorption from the intestine is important for proper mineralization of the bone. The second major function of vitamin D is involvement in the maturation of osteoclasts, which resorb calcium from the bones. (See Pathophysiology and Etiology.)
The term "vitamin D" refers to either vitamin D2 or vitamin D3. Vitamin D3, also known as cholecalciferol, is either made in the skin or obtained in the diet from fatty fish. Vitamin D2, also known as ergocalciferol, is obtained from irradiated fungi, such as yeast. Vitamin D2 and vitamin D3 are used to supplement food products or are contained in multivitamins. (See Treatment and Medication.)
Past studies suggested that vitamin D3 may be more effective than vitamin D2 in establishing normal vitamin D stores. [11, 12] However, a study by Holick and colleagues demonstrated that vitamin D2 and vitamin D3 appear to be equipotent in raising 25(OH)D concentrations when they are given in daily doses of 1000 IU. [13]
Vitamin D deficiency during pregnancy affects offspring. In a community-based study of 901 mother-and-offspring pairs, researchers found that maternal vitamin D deficiency (here, serum 25-hydroxyvitamin D < 20 ng/mL) at 18 weeks' pregnancy was associated with impaired lung development at age 6 years in offspring, neurocognitive difficulties at age 10 years, increased risk of eating disorders in adolescence, and lower peak bone mass at age 20 years. [14, 15, 16]
Findings suggest that vitamin D plays an active role in fetal development, particularly the development of the brain, lungs, and bones. Given increased risk of vitamin D deficiency in pregnancy and the impact of vitamin D deficiency on neonatal outcomes, the Endocrine Society recommends "empiric vitamin D supplementation during pregnancy." [6]
Physiology
The production of vitamin D3 in the skin involves a series of reactions initiating with 7-dehydrocholesterol. Upon exposure to ultraviolet-B (UVB) radiation between the wavelengths of 290-315 nm, 7-dehydrocholesterol is converted to previtamin D3, which is then converted to vitamin D3 after a thermally induced isomerization reaction in the skin. From the skin, newly formed vitamin D3 enters the circulation by binding to vitamin D binding protein (DBP). In order to become active, vitamin D requires 2 sequential hydroxylations to form 1,25-dihydroxyvitamin D (1,25[OH]2 D).
Vitamin D is initially hydroxylated in the 25 position by the hepatic microsomal and/or mitochondrial enzyme vitamin D 25-hydroxylase. The second hydroxylation occurs in the kidney and is performed by the P450 enzyme 25-hydroxyvitamin D-1 alpha-hydroxylase.
Upon entering the cell, the 1,25(OH)2 D hormone binds to the vitamin D receptor (VDR). The bound vitamin D receptor then forms a heterodimer with the retinoic acid X receptor (RXR). This heterodimer then goes to the nucleus to bind deoxyribonucleic acid (DNA) and increases transcription of vitamin D–related genes.
Pathophysiology
Inadequate circulating 25(OH)D is associated with elevated parathyroid hormone (PTH); this condition is called secondary hyperparathyroidism. The rise in PTH may result in increased mobilization of calcium from the bone, which leads to decreased mineralization of the bone.
Of note, prolonged exposure to the sun does not cause vitamin D toxicity. This is because after prolonged UVB radiation exposure, the vitamin D made in the skin is further degraded to the inactive vitamin D metabolites tachysterol and lumisterol.
As strongly suggested by genetic, molecular, cellular, and animal studies, extraskeletal effects related to vitamin D signaling include roles in cell proliferation, immune and muscle function, skin differentiation, and reproduction, with vitamin D having vascular and metabolic actions as well. Observational studies have pointed to a relationship between poor vitamin D status and almost all diseases connected to these extraskeletal influences. However, while randomized, controlled trials and Mendelian randomization studies have indicated that vitamin D supplementation can lower the incidence of some disorders, only mixed conclusions on the matter have been reached globally. [17]
Etiology
Vitamin D deficiency can result from the following:
-
Inadequate exposure to sunlight - This causes a deficiency in cutaneously synthesized vitamin D; adults in nursing homes or health care institutions are at a particularly high risk. [18]
-
Minimal amounts of vitamin D in human breast milk - The American Academy of Pediatrics recommends vitamin D supplementation starting at age 2 months for infants fed exclusively with breast milk. [21]
-
Medications - Some medications are associated with vitamin D deficiency; drugs such as Dilantin, phenobarbital, and rifampin can induce hepatic p450 enzymes to accelerate the catabolism of vitamin D.
Epidemiology
Occurrence in the United States
Vitamin D insufficiency is highest among people who are elderly, institutionalized, or hospitalized. In the United States, 60% of nursing home residents [22] and 57% of hospitalized patients [23] were found to be vitamin D deficient.
However, vitamin D insufficiency is not restricted to the elderly and hospitalized population; several studies have found a high prevalence of vitamin D deficiency among healthy, young adults. A study determined that nearly two thirds of healthy, young adults in Boston were vitamin D insufficient at the end of winter. [24]
A study by Cui et al using the National Health and Nutrition Examination Survey (NHANES), 2001-2018, found severe and moderate vitamin D deficiency in the United States to have a weighted prevalence of 2.6% and 22.0%, respectively, with a weighted prevalence of 40.9% for insufficiency. (In the study, severe deficiency was defined as less than 10 ng/mL; moderate deficiency, as between 10 and 20 ng/mL; and insufficiency, as between 20 and 30 ng/mL. The parameters for vitamin D deficiency/insufficiency vary between sources.) [25]
Vitamin D status may fluctuate throughout the year, with the highest serum 25(OH)D level occurring after the summer and the lowest serum 25(OH)D concentrations after winter. A study by Shoben et al demonstrated that mean serum 25(OH)D concentrations can vary as much as 9.5 ng/mL. Factors such as male sex, higher latitude, and greater physical activity levels were found to be associated with greater differences in serum 25(OH)D concentrations in winter and summer. [26]
International occurrence
A greater prevalence of vitamin D deficiency exists in Middle Eastern countries. A study of 316 young adults aged 30-50 years from the Middle East showed that 72.8% had 25(OH)D values of less than 15 ng/dL (defined, at that time, as severely deficient, again pointing to the variation in vitamin D thresholds between sources). This was significantly more common in women than in men (83.9% vs 48.5%, respectively). The difference between sexes probably reflected the cultural and religious practices leading to less skin exposure in women than in men. [27, 28, 29, 30]
Race-related demographics
Darker skin interferes with the cutaneous synthesis of vitamin D. A study by Holick and coauthors demonstrated that non-Hispanic Black subjects require 6 times the amount of UV radiation necessary to produce a serum vitamin D concentration similar to that found in non-Hispanic White subjects. [31] The explanation for the increased radiation necessary to increase vitamin D levels is that melanin absorbs UV radiation.
The decreased efficacy of vitamin D production by darker-pigmented skin explains the higher prevalence of vitamin D insufficiency among darker-skinned adults. Dawson-Hughes and colleagues demonstrated that in Boston, 73% of elderly Black subjects were vitamin D insufficient, compared with 35% of elderly non-Hispanic Whites. [32]
In a large survey of 1500 healthy Black women younger than 50 years, 40% were vitamin D insufficient or deficient (25[OH]D < 16ng/mL), compared with 4% of 1400 White women in that study. [33]
Age-related demographics
Vitamin D production in the skin declines with advancing age, making elderly populations more dependent on dietary vitamin D. For the average older person, higher dietary intake of vitamin D may be required to achieve optimal serum levels of 25(OH)D. [34]
Prognosis
The treatment of vitamin D insufficiency can decrease the risk of hip and nonvertebral fractures. [35, 36] A meta-analysis by Boonen et al of postmenopausal women and of men aged 50 years or older reporting a risk of hip fracture found that oral vitamin D supplementation reduced the risk of hip fractures by 18% when vitamin D and calcium were taken together. [37] Most of the trials that demonstrated the antifracture efficacy of vitamin D used approximately 800 IU of vitamin D3. The minimum 25(OH)D level at which antifracture efficacy was observed was 30 ng/mL, suggesting a threshold for optimal levels of 25(OH)D for fracture protection.
Results from another meta-analysis, evaluating the efficacy of oral vitamin D supplementation in the prevention of hip and other nonvertebral bone fractures in individuals aged 65 years or older, indicated that vitamin D offers dose-dependent fracture protection. [38] The analysis, by Bischoff-Ferrari et al, took into account 12 double-blind, randomized, controlled trials (RCTs) for nonvertebral fractures (n = 42,279) and 8 RCTs for hip fractures (n = 40,886), comparing the results obtained from the use of oral vitamin D (with or without calcium) with those derived from the administration of calcium alone and from placebo use.
In this study, doses of more than 400 IU/day were found to reduce fractures by at least 20% in individuals aged 65 years or older. [38] In contrast to the Boonen study, the investigators maintained that these effects were independent of calcium supplementation.
Vitamin D insufficiency contributes to osteoporosis by decreasing intestinal calcium absorption. [10, 39] Treatment of vitamin D deficiency has been shown to improve bone mineral density. [40, 41] An analysis of the Third National Health and Nutrition Examination Survey (NHANES III) demonstrated a positive correlation between circulating 25(OH)D levels and bone mineral density. [42]
Vitamin D supplementation has been associated with a reduction in falls and improved muscle strength in the elderly. A meta-analysis demonstrated that vitamin D supplementation resulted in a reduction in falls of about 22% in ambulatory and institutionalized elderly subjects, as compared with controls. [43, 44] Another meta-analysis examining muscle strength associated with vitamin D supplementation found significant improvement in reduced postural sway, timed up-and-go test results, and lower extremity strength in a pooled analysis of 13 studies. [45]
Epidemiologic data suggest that vitamin D deficiency places adults at risk for developing cancer; [46, 47, 48, 49, 50] these apparently include breast, colon, and prostate cancer. [51, 52]
A retrospective study by Grasemann et al indicated that parathyroid hormone (PTH) elevation in vitamin D deficiency may be associated with poor event-free survival in pediatric patients with cancer. Looking at PTH and 25(OH)D levels, highest for the former and lowest for the latter, over a 5-year period (starting at diagnosis) for 1286 pediatric patients with cancer, multivariate analysis found that high PTH could be linked to inferior event-free survival in children with primary malignant brain tumors, embryonal malignancies, and lymphatic malignancies. [53]
However, the majority of randomized, controlled trials looking into the link between vitamin D supplements and cancer prevention have reported that no such relationship exists, with supplementation not reducing “the risk of developing cancer overall or of developing specific cancers.” Results of randomized, controlled trials have varied with regard to whether vitamin D supplements can lower the mortality risk from cancer. Moreover, many of the individuals taking part in the vitamin D/cancer mortality studies had adequate vitamin D levels, which has led to research on whether supplements for people with lower levels can impact mortality risk. [54]
A study by Manson et al to evaluate the efficacy of vitamin D supplementation and omega-3 fatty acids in lowering the risk of invasive cancer and cardiovascular disease found no such reduction associated with the administration of vitamin D. The report, which included 25,871 participants (males aged 50 years or older and females aged 55 years or older) and had a median follow-up period of 5.3 years, determined that compared with a placebo group, there was no decrease in the incidence of breast, prostate, or colorectal cancer, or in myocardial infarction, stroke, or cardiovascular-related death, among subjects who consumed 2000 IU per day in supplementary vitamin D. [55]
Observational studies have demonstrated more positive results in cancer risk reduction, indicating that vitamin D supplements can decrease colorectal and, less successfully, bladder cancer risks. [54]
Vitamin D insufficiency may increase the risk for type I and type II diabetes mellitus. [34, 56] In NHANES III, lower vitamin D status was associated with higher fasting glucose and 2-hour glucose after an oral glucose tolerance test. [57] Furthermore, vitamin D supplementation in adults has been associated with improved insulin sensitivity in several small, case-control studies. [56]
Joergensen et al determined that vitamin D deficiency in type 1 diabetes may predict all causes of mortality but not development of microvascular complications. [58] The contribution of vitamin D deficiency to mortality must be mediated by nonvascular mechanisms.
A study by Li et al indicated that vitamin D deficiency (serum 25-hydroxyvitamin D < 12 ng/mL) is related to an increased stroke risk in adults, with an association also found between higher vitamin D levels and a reduced stroke risk. The study, on adults aged 20 years or older, found that evidence for the relationship between high serum 25-hydroxyvitamin D levels and decreased stroke risk was particularly strong among females below age 50 years. [59]
Low levels of vitamin D have also been linked to increased cardiovascular disease (CVD) biomarkers in older adults. In an observational study of 957 hypertensive older adults, vitamin D deficiency (here, < 10 ng/mL) was associated with higher levels of biomarkers linked with CVD and conditions such as multiple sclerosis and rheumatoid arthritis. [60, 61, 62] Individuals deficient in vitamin D had significantly higher levels of the inflammatory biomarkers interleukin-6 (IL-6) and C-reactive protein (CRP), and higher IL-6:IL-10 and CRP:IL-10 ratios compared with subjects who had serum vitamin D levels > 30 ng/mL. [60, 61]
A meta-analysis evaluated the effect of vitamin D supplementation (using a mean supplementation dosage of about 500 IU daily) on all-cause mortality in 18 randomized controlled trials and found a 7% relative risk reduction for death. [63] Severe vitamin D deficiency (25(OH)D < 10 ng/mL) has been associated with increased in-hospital mortality in patients admitted for acute coronary syndrome. [64]
A Cochrane Review of 50 randomized, controlled trials that included more than 94,000 individuals, primarily elderly women, found that vitamin D3 supplementation decreased mortality. Other forms of vitamin D, including vitamin D2, calcitriol, and alpha-calcidiol, did not reduce mortality. [65]
COVID-19
Low vitamin D in patients with coronavirus disease 2019 (COVID-19) reflects a heightened inflammatory state and possibly could be used as a surrogate marker for high risk of severe disease. [66]
A retrospective case-control study by Jude et al indicated that vitamin D deficiency can be linked to a greater likelihood of hospitalization in patients with COVID-19. Results from a primary cohort revealed that in hospitalized patients with COVID-19, the median serum level of 25(OH)D was 35.0 nmol/L, compared with 50.0 nmol/L in nonhospitalized individuals with COVID-19. The investigators suggested that the risk of hospitalization for COVID-19 could be reduced via widespread testing for and treatment of serum 25(OH)D insufficiency or deficiency. [67]
A retrospective observational study by De Smet et al indicated that patients with COVID-19 who have vitamin D deficiency at hospital admission have an almost four-fold greater chance of mortality from COVID-19. This increase in the study occurred independently of age, ethnicity, the presence of chronic lung disease, or the degree of lung damage (as determined through chest computed tomography [CT] scan severity score). [68, 69]
A retrospective, single-institution study by Dror et al of hospitalized patients with COVID-19 found that those who had a 25(OH)D level of less than 20 ng/mL prior to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, had a 14-fold greater likelihood of severe or critical COVID-19 than did those who had a 25(OH)D level of 40 ng/mL or greater before infection. Moreover, the mortality rate during hospitalization in patients with pre-infection vitamin D deficiency was 25.6%, compared with 5.0% in those with 40 ng/mL or more of 25(OH)D. [70]
A study by Jolliffe et al indicated that in persons with suboptimal vitamin D status, providing vitamin D supplementation through a test-and-treat approach does not protect against COVID-19. The baseline prevalence of suboptimal vitamin D prevalence was high in the study group, which consisted of individuals aged 16 years or older. Results regarding the incidence and severity of acute COVID-19 or of prolonged COVID-19 symptoms did not differ significantly between individuals who were not offered supplementation and those who were, no matter whether lower- or higher-dose supplementation was provided. (However, the investigators stated that there was a low prevalence of profound vitamin D deficiency at baseline within the group, and that consequently the study could not determine whether persons with this status might benefit from supplementation.) [71]
-
Findings in patients with rickets.
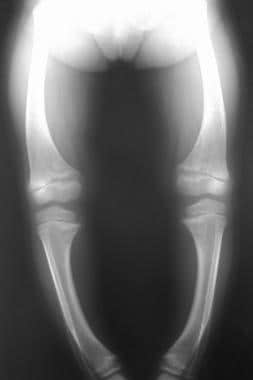
-
Radiograph in a 4-year-old girl with rickets depicts bowing of the legs caused by loading.
-
Anteroposterior and lateral radiographs of the wrist of an 8-year-old boy with rickets demonstrates cupping and fraying of the metaphyseal region.