Background
Mucormycosis, previously called zygomycosis, refers to several different diseases caused by infection with fungi belonging to the order Mucorales. Rhizopus species are the most causative organisms of this group. In descending order, the other genera with mucormycosis-causing species include Mucor, Cunninghamella, Apophysomyces, Lichtheimia (formerly Absidia), Saksenaea, Rhizomucor, and other species. [1, 2]
Most mucormycosis infections are life-threatening. Risk factors such as diabetic ketoacidosis and neutropenia are present in most cases. Severe sinusitis, complicated by brain abscess, is the most common presentation. Pulmonary, cutaneous, and gastrointestinal (GI) infections also are recognized.
Successful mucormycosis treatment requires correction of the underlying risk factor(s), antifungal therapy (traditionally with a polyene), and aggressive surgery.
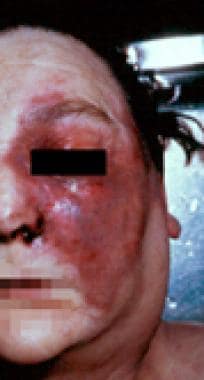 Postmortem photograph of a woman with diabetes and left rhinocerebral mucormycosis complicating ketoacidosis. Rhizopus oryzae was the causative organism. Note the orbital and facial cellulitis and the black nasal discharge. Courtesy of A Allworth, MD, Brisbane, Australia.
Postmortem photograph of a woman with diabetes and left rhinocerebral mucormycosis complicating ketoacidosis. Rhizopus oryzae was the causative organism. Note the orbital and facial cellulitis and the black nasal discharge. Courtesy of A Allworth, MD, Brisbane, Australia.
See also Pediatric Mucormycosis and Rhinocerebral Mucormycosis.
Etiology and Pathophysiology
Risk factors
Immunocompromising conditions are the main risk factors for mucormycosis. Patients with uncontrolled diabetes mellitus, especially those with ketoacidosis, are at high risk. Other high-risk groups include patients with cancer, especially those who are neutropenic and those receiving broad-spectrum antibiotics, and individuals receiving immunosuppressive agents, including oral or intravenous steroids and tumor necrosis factor (TNF)-alpha blockers (patients with rheumatoid disorders). In addition, patients with hematologic cancer who have opportunistic cytomegalovirus (CMV) infections and graft versus host disease (GVHD) are at an increased risk. Prior receipt of voriconazole is another risk factor for mucormycosis. [3]
Extreme malnutrition is linked to mucormycosis, especially the gastrointestinal (GI) form. Iron is a growth stimulant for Mucorales; older iron chelators such as deferoxamine and all causes of iron overload are additional risk factors for mucormycosis. Trauma and the use of contaminated medical supplies over wounds are associated with cutaneous mucormycosis. Additionally, nonsterile tape and contaminated wooden splints are other risk factors for cutaneous diseases. [4, 5] Such cases are associated with trauma/surgery or the presence of a preexisting wound or intravascular line. Patients with burns and those who use intravenous drugs are at a higher risk.
Some patients with mucormycosis have no identifiable risk factors. [6, 7] Invasive mucormycosis also has been associated with health-care associated outbreaks and natural disasters. [8, 9, 10, 11, 12]
Pathophysiology
Mucorales are ubiquitous fungi that are commonly found in soil and in decaying matter. Rhizopus can be found in moldy bread. The major route of infection is via inhalation of conidia; other routes include ingestion and traumatic inoculation (see the images below). Ingestion leads to GI disease and occurs primarily among malnourished patients but also can occur after ingesting non-nutritional substances (pica).
Mucoraceae are molds in the environment that become hyphal forms in tissues. Once the spores begin to grow, fungal hyphae invade blood vessels, producing tissue infarction, necrosis, and thrombosis. When spores are deposited in the nasal turbinates, rhinocerebral disease develops (see Rhinocerebral Mucormycosis); when spores are inhaled into the lungs, pulmonary disease develops; when ingested, GI disease ensues; and when the agents are introduced through interrupted skin, cutaneous disease develops.
The virulence factor involved in the pathogenesis of Mucorales is high affinity iron permease (FTR1), allowing Mucorales survival in iron-poor environments. [13, 14, 15] Spore coat protein (Cot H) present on the surface of Mucorales results in impaired host defense [13] ; ADP-ribosylation factor plays a significant role in the growth of Mucorales. [13] Neutrophils are the key host defense against these fungi; thus, individuals with neutropenia or neutrophil dysfunction (eg, diabetes, steroid use) are at highest risk. [16] Few cases of mucormycosis have been reported in patients with acquired immunodeficiency syndrome (AIDS), suggesting that the host defense against this infection is not primarily mediated by cellular immunity.
Mucormycosis should be considered in the differential diagnosis of a necrotic-appearing wound or one with an inadequate response to antibiotic treatment. [17, 18, 19] External factors, such as toxins produced by bacterial endosymbiosis, can lead to endothelial disruption, resulting in fungal virulence. [13, 20, 21] Additionally, voriconazole exposure by a unknown mechanism and not due to selective pressure, allows for invasive mucormycosis. [13, 22]
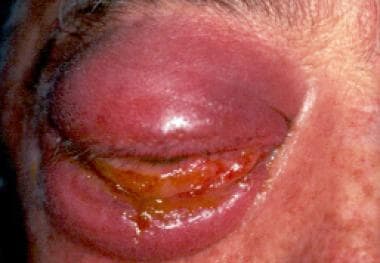 The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Note the proptosis. Courtesy of A Allworth, MD, Brisbane, Australia.
The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Note the proptosis. Courtesy of A Allworth, MD, Brisbane, Australia.
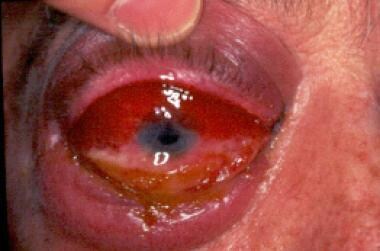 The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Chemosis is shown in this photograph. Internal and external ophthalmoplegia, no light perception, and afferent pupil defect were present, which is consistent with orbital apex syndrome. Courtesy of A Allworth, MD, Brisbane, Australia.
The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Chemosis is shown in this photograph. Internal and external ophthalmoplegia, no light perception, and afferent pupil defect were present, which is consistent with orbital apex syndrome. Courtesy of A Allworth, MD, Brisbane, Australia.
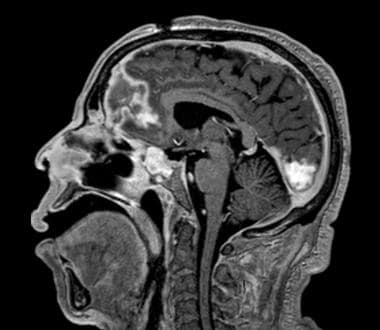 Brain MRI (sagittal view) in a patient with uncontrolled diabetes who presented with progressive right eye pain and facial swelling. He underwent multiple surgeries to control rhinocerebral Mucor infection, including partial right frontal lobectomy and right orbital exenteration. He was treated with amphotericin B and his diabetes mellitus was controlled. The disease did not progress, and long-term isavuconazole therapy was initiated for salvage/maintenance therapy.
Brain MRI (sagittal view) in a patient with uncontrolled diabetes who presented with progressive right eye pain and facial swelling. He underwent multiple surgeries to control rhinocerebral Mucor infection, including partial right frontal lobectomy and right orbital exenteration. He was treated with amphotericin B and his diabetes mellitus was controlled. The disease did not progress, and long-term isavuconazole therapy was initiated for salvage/maintenance therapy.
Epidemiology
United States
Mucormycosis is rare, and its incidence is difficult to calculate accurately. Further, since mucormycosis is not a reportable disease, the true incidence is unknown. An estimated burden of mucormycosis in United States is 3.0 cases per 1 million people, increased from 1.75 cases per 1 million people in the United States annually. [23, 24, 25] A retrospective study among 560 hospitals with 104 million patients estimated the prevalence of mucormycosis-related hospitalizations at 0.12 per 10,000 discharges. [25, 26] A prospective surveillance for invasive fungal infections conducted at 23 transplant centers from 2001-2006 reported an incidence of 0.29% for hematopoietic stem cell transplant (HSCT) and 0.07% for solid organ transplant. [25, 26] A rise in mucormycosis incidence was seen in patients with hematological malignancy per 100 autopsies, from 0.006 cases (1989-1993) to 0.018 cases in (2004-2008). [25, 27]
The incidence of mucormycosis appears to be increasing secondary to rising numbers of immunocompromised persons. There are also increasing reports of breakthrough mucormycosis in the setting of antifungal prophylaxis or treatment (eg, voriconazole, echinocandins) that is effective against most fungi, such as Aspergillus, but not mucormycosis. With improvements in early diagnosis via modalities such as matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), polymerase chain reaction (PCR), and 18s rRNA sequencing, it has been suggested that mucormycosis is the cause of more than 10% of invasive fungal infections. [28, 29]
Additionally, several cases of cutaneous mucormycosis due to the mucormycete Apophysomyces trapeziformis were described among wound-injured victims from a tornado. [18, 30, 31]
International
A rise in mucormycosis cases has been seen globally; the estimate of mucormycosis rose to 910,000 cases globally. [25, 32, 33] Increased cases were noted in Asia and Europe. A multicenter study from Spain estimating prevalence of mucormycosis reported 0.43 cases per million population/year. [25, 34] Similarly, a population-based study conducted nationwide in France reported an increase in prevalence in 1997 to 2006, 0.7 cases to 1.2 per million cases, respectively. [25, 35] A single centre study from India reported a rise in mucormycosis cases from 24.7 cases to 89 cases per year. [25, 36] A study conducted in multiple ICUs in India reported high burden of mucormycosis, detected in 14.4% of patients. [25, 37]
Prognosis
Prognosis and survival depend on early diagnosis and timely initiation of treatment. [38] Mortality of mucormycosis varies between 40-80%, despite advances in treatment; mortality depends on the site of infection. [39] Higher mortality of 80% is seen in among patients with disseminated disease to CNS [39] ; rhino-orbital-cereberal mucormycosis has a mortality rate of 25-62%. Among patients with orbital involvement, early diagnosis through nasal endoscopic examination and rapid initiation of treatment are important in improving disease specific mortality. [13, 40, 41, 42] Rapid diagnosis and early surgical debridement lead to lower mortality; this can be achieved among patients with localized sinus and skin disease. [39, 43, 44]
Mortality also is high among patients with gastrointestinal manifestations, as diagnosis often is achieved late due to non-specific presentation. [39, 45] Pulmonary mucormycosis carries a mortality rate of 57.1%, however mortality has improved over time due to combination of medical and surgical treatment. [46]
COVID-19 and Mucormycosis
The COVID-19 pandemic, caused by severe SARS-CoV-2, has affected 177 million people worldwide and accounted for 3.82 million deaths as of June 2021. COVID-19 disease can be complicated by secondary bacterial infection; however, reports of invasive fungal infection (COVID-19-associated pulmonary aspergillosis, pneumocystosis, and mucormycosis) are rising. [47] The incidence of COVID-19-associated mucormycosis (CAM) has increased in India, with more than 20,000 cases reported. [48, 49, 50, 51]
COVID-19 has a predisposition to cause extensive lung damage, which promotes colonization and infection of invasive fungi of airway, sinuses, and lung. [48, 52, 53, 54, 55] Alteration in T cell immunity, use of steroids, and broad-spectrum antibiotics are additional risk factors for acquiring invasive fungal infection. [48, 52, 53, 54, 55] COVID-19 disrupts iron metabolism, resulting in high ferritin state and increasing intracellular iron, which causes tissue damage. This causes more iron to be released into the circulation, and it is this increase in free iron that is a risk factor for mucormycosis. [47]
Diabetes mellitus previously has been described in this chapter as a risk factor for mucormycosis. Patients with diabetes and infected with SARS-CoV2 are at increased risk for mucormycosis. High expression of ACE-2 receptors in pancreatic islets caused by SARS-CoV-2 results in insulin resistance leading to poorly controlled diabetes, further increasing the risk for mucormycosis.
A retrospective study conducted in India from September-December 2020 reported a 2.0 fold rise in 2020 compared to 2019 of COVID-19 mucormycosis, with prevalence of 0.27% among hospitalized COVID-19 patients; the most common clinical manifestation was rhino-orbital form. [56] Case fatality at 12 weeks was 45.7% with no difference in mortality among COVID-19 and non-COVID-19 patients. [56]
Please refer to the Treatment section for details on medication dosing.
-
Postmortem photograph of a woman with diabetes and left rhinocerebral mucormycosis complicating ketoacidosis. Rhizopus oryzae was the causative organism. Note the orbital and facial cellulitis and the black nasal discharge. Courtesy of A Allworth, MD, Brisbane, Australia.
-
The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Note the proptosis. Courtesy of A Allworth, MD, Brisbane, Australia.
-
The right eye of an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Chemosis is shown in this photograph. Internal and external ophthalmoplegia, no light perception, and afferent pupil defect were present, which is consistent with orbital apex syndrome. Courtesy of A Allworth, MD, Brisbane, Australia.
-
An immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. A surgical field of this patient is shown. Excision of the right orbit, maxillary antrum, nasal cavity, sphenoid sinus, and infratemporal fossa has taken place. The tissue was infarcted. Courtesy of A Allworth, MD, Brisbane, Australia.
-
An immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Picture of the patient after successful treatment with repeated surgical debridement and high-dose liposomal amphotericin B. Courtesy of A Allworth, MD, Brisbane, Australia.
-
Histologic findings from an immunocompetent man who sustained a high-pressure water jet injury, resulting in rhinocerebral mucormycosis. Traumatic inoculation of Apophysomyces elegans was the pathogenetic mechanism. Findings show the typical Mucorales hyphae on Grocott methenamine-silver staining. The hyphae are the dark structures with budlike, right-angle hyphae. Courtesy of A Allworth, MD, Brisbane, Australia.
-
Chest computed tomography (CT) scan showing pulmonary mucormycosis with left basal consolidation and widespread nodules due to Rhizopus oryzae infection. The patient was receiving cytotoxic chemotherapy for myelodysplastic syndrome and had iron overload from numerous blood transfusions.
-
Chest computed tomography (CT) scan showing pulmonary mucormycosis with left basal consolidation and widespread nodules due to Rhizopus oryzae infection. The patient was receiving cytotoxic chemotherapy for myelodysplastic syndrome and had iron overload from numerous blood transfusions. This CT scan of the patient shows resolution of pulmonary mucormycosis after 5 months of antifungal treatment.
-
Brain MRI (sagittal view) in a patient with uncontrolled diabetes who presented with progressive right eye pain and facial swelling. He underwent multiple surgeries to control rhinocerebral Mucor infection, including partial right frontal lobectomy and right orbital exenteration. He was treated with amphotericin B and his diabetes mellitus was controlled. The disease did not progress, and long-term isavuconazole therapy was initiated for salvage/maintenance therapy.
-
Rhino-orbital-cerebral mucormycosis in a patient with SARS-CoV-2 infection. Courtesy of Dr Sujata Rege.
-
Pulmonary mucormycosis in a patient with SARS-CoV-2 infection. Courtesy of Dr Sujata Rege.







