Practice Essentials
Rectal cancer is a disease in which cancer cells form in the tissues of the rectum; colorectal cancer occurs in the colon or rectum. Adenocarcinomas comprise the vast majority (98%) of colon and rectal cancers; more rare rectal cancers include lymphoma (1.3%), carcinoid (0.4%), and sarcoma (0.3%).
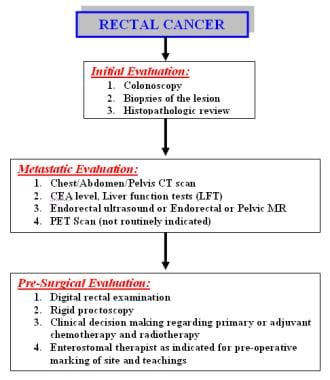
The incidence and epidemiology, etiology, pathogenesis, and screening recommendations are common to both colon cancer and rectal cancer. The image below depicts the staging and workup of rectal cancer.
Signs and symptoms
Bleeding is the most common symptom of rectal cancer, occurring in 60% of patients. However, many rectal cancers produce no symptoms and are discovered during digital or proctoscopic screening examinations.
Other signs and symptoms of rectal cancer may include the following:
-
Change in bowel habits (43%): Often in the form of diarrhea; the caliber of the stool may change; there may be a feeling of incomplete evacuation and tenesmus
-
Occult bleeding (26%): Detected via a fecal occult blood test (FOBT)
-
Abdominal pain (20%): May be colicky and accompanied by bloating
-
Back pain: Usually a late sign caused by a tumor invading or compressing nerve trunks
-
Urinary symptoms: May occur if a tumor invades or compresses the bladder or prostate
-
Malaise (9%)
-
Pelvic pain (5%): Late symptom, usually indicating nerve trunk involvement
-
Emergencies such as peritonitis from perforation (3%) or jaundice, which may occur with liver metastases (< 1%)
See Presentation for more detail.
Diagnosis
Perform physical examination with specific attention to the size and location of the rectal tumor as well as to possible metastatic lesions, including enlarged lymph nodes or hepatomegaly. In addition, evaluate the remainder of the colon.
Examination includes the use of the following:
-
Digital rectal examination (DRE): The average finger can reach approximately 8 cm above the dentate line; rectal tumors can be assessed for size, ulceration, and presence of any pararectal lymph nodes, as well as fixation to surrounding structures (eg, sphincters, prostate, vagina, coccyx and sacrum); sphincter function can be assessed
-
Rigid proctoscopy: This examination helps to identify the exact location of the tumor in relation to the sphincter mechanism
Screening tests
Screening tests may include the following:
-
Guaiac-based FOBT
-
Stool DNA screening (SDNA)
-
Fecal immunochemical test (FIT)
-
Rigid proctoscopy
-
Flexible sigmoidoscopy (FSIG)
-
Combined glucose-based FOBT and flexible sigmoidoscopy
-
Double-contrast barium enema (DCBE)
-
Computed tomography (CT) colonography
-
Fiberoptic flexible colonoscopy (FFC)
Laboratory studies
Routine laboratory studies in patients with suspected rectal cancer include the following:
-
Complete blood count
-
Serum chemistries
-
Liver and kidney function tests
-
Carcinoembryonic antigen (CEA) test
-
Histologic examination of tissue specimens
Imaging studies
If metastatic (local or systemic) rectal cancer is suspected, the following radiologic studies may be obtained:
-
CT scanning of the chest, abdomen, and pelvis
-
Pelvic magnetic resonance imaging (MRI): Preferred study
-
Endorectal ultrasonography: May be used if pelvic MRI is not readily available
-
Positron emission tomography (PET) scanning: Not routinely indicated
See Workup for more detail.
Management
A multidisciplinary approach that includes surgery, medical oncology, and radiation oncology is required for optimal treatment of patients with rectal cancer. Surgical technique, use of radiotherapy, and method of administering chemotherapy are important factors.
Considerations with respect to surgical technique include the intent of surgery (curative or palliative), restoration of bowel function, and preservation of anal continence and genitourinary functions. Ideally, the intent is achievement of a cure, because the risk of pelvic recurrence is high in patients with rectal cancer, and locally recurrent rectal cancer has a poor prognosis.
Surgery
Radical resection of the rectum is the mainstay of therapy. The choice of surgical procedure is based on the size, location, extent, and grade of the rectal carcinoma. Operative management of rectal cancer may include the following:
-
Transanal excision: For early-stage cancers in a select group of patients
-
Transanal endoscopic microsurgery: A form of local excision that uses a special operating proctoscope that distends the rectum with insufflated carbon dioxide and allows the passage of dissecting instruments
-
Endocavity radiotherapy: Delivered under sedation via a special proctoscope in the operating room
-
Sphincter-sparing procedures: Low anterior resection, coloanal anastomosis, abdominal perineal resection
Adjuvant medical management
Adjuvant medical therapy may include the following:
-
Adjuvant radiation therapy
-
Intraoperative radiation therapy
-
Preoperative or postoperative chemotherapy
-
Preoperative or postoperative chemoradiation therapy
-
Thermal ablation or radioembolization of liver metastases
Pharmacotherapy
Chemotherapy for rectal cancer may be given preoperatively or postoperatively, either alone or in combination with radiation therapy. The following agents may be used in the management of rectal cancer:
-
Antineoplastic agents (eg, fluorouracil, vincristine, leucovorin, irinotecan, oxaliplatin, cetuximab, bevacizumab, panitumumab)
-
Biologic agents (eg, cetuximab, encorafenib, fruquintinib): As targeted therapy, typically as part of combination regimens, for unresectable or metastatic rectal cancer
See Treatment and Medication for more detail.
Background
Colon and rectal cancer incidence was negligible before 1900. The incidence of colorectal cancer rose dramatically following economic development and industrialization. Currently, colorectal cancer is the third most common cancer in both men and women in the United States. [1]
Adenocarcinomas comprise the vast majority (98%) of colon and rectal cancers. Other rare rectal cancers, including carcinoid (0.4%), lymphoma (1.3%), and sarcoma (0.3%), are not discussed in this article. Squamous cell carcinomas may develop in the transition area from the rectum to the anal verge and are considered anal carcinomas. Very rare cases of squamous cell carcinoma of the rectum have been reported. [2, 3]
Approximately 20% of colorectal cancers develop in the cecum, another 20% in the rectum, and an additional 10% in the rectosigmoid junction. Approximately 25% of colon cancers develop in the sigmoid colon. [2]
The incidence and epidemiology, etiology, pathogenesis, and screening recommendations are common to both colon cancer and rectal cancer. These areas are addressed together.
Anatomy
The surgical definition of the rectum differs from the anatomical definition. Surgeons define the rectum as starting at the level of the sacral promontory. The National Comprehensive Cancer Network (NCCN) bases its definition on magnetic resonance imaging (MRI) results, stating that "the rectum lies below a virtual line from the sacral promontory to the upper edge of the symphysis as determined by MRI." [4] Anatomists define the rectum as starting at the level of the 3rd sacral vertebra. Therefore, the measured length of the rectum varies from 12 cm to 15 cm.
The rectum differs from the rest of the colon in that the outer layer consists of longitudinal muscle. The rectum contains three folds, namely the valves of Houston. The superior (at 10 cm to 12 cm) and inferior (at 4 cm to 7 cm) folds are located on the left side and the middle fold (at 8 cm to 10 cm) is located at the right side.
European Society for Medical Oncology (ESMO) guidelines define rectal cancer as cancer located within 15 cm of the anal verge. [5] ESMO guidelines categorize rectal cancers as low, medium, or high, according to the distance of their distal edge from the anal verge. Location, based on rigid proctoscopy or flexible endoscopy, is as follows:
-
Low: Up to 5 cm from anal verge
-
Medium: > 5 to 10 cm from anal verge
-
High: > 10 to 15 cm from anal verge
In contrast, tumor location based on MRI, which uses anorectal junction rather than anal verge as a landmark, is as follows [5] :
-
Low: ≤ 4 cm from anorectal junction
-
Medium: 4 to 8 cm from anorectal junction
-
High: > 8 to 12 cm from anorectal junction
Tumor location within the rectum can influence treatment decisions. [5] For example, NCCN lines list tumor location within 8 cm of the anal verge as one of the criteria for use of transanal local resection. [4]
Computed tomography or MRI is the most common modality to define the anatomic rectum. The variations in the definition of anatomic rectum result in variations in the treatment options, most importantly determining whether neoadjuvant chemoradiotherapy is indicated. Neoadjuvant therapy has significant consequences in the functional and oncologic outcomes of patients.
The following features are used to define the rectum apart from the sigmoid colon [6] :
-
The sacral promontory
-
The third valve of Houston
-
The coalescence of tenia of sigmoid
-
The transition from sigmoid mesocolon to mesorectum
-
Loss of appendiceal epiploicae
-
Anterior peritoneal reflection
In order to standardize treatment of colorectal cancer, an international group of colorectal experts established a consensus definition of the anatomic rectum, using the Delphi technique. This consensus meeting came up with new terminology, the sigmoid takeoff, which defines the junction of sigmoid colon and rectum and the junction of sigmoid mesocolon and mesorectum radiologicallt. [6] On imaging, the sigmoid take-off is where the sigmoid sweeps away from the sacrum, with ventral projection in the axial plane and/or horizontal projection in the sagittal plane. [7] Although there are many publications on this topic in the literature, all rectal surgeons should familiarize themselves with the Delphi consensus article, as well as "The 'Holy Plane' of rectal surgery", which defines an optimal dissection plane around rectal tumors in terms of the pelvic fascia. [6, 8]
Pathophysiology
The mucosa in the large intestine regenerates approximately every 6 days. Crypt cells migrate from the base of the crypt to the surface, where they undergo differentiation and maturation, and ultimately lose the ability to replicate.
The vast majority of colorectal cancers are adenocarcinomas. Colonic adenomas precede adenocarcinomas. Approximately 10% of adenomas will eventually develop into adenocarcinomas. This process may take up to 10 years. [2] The adenoma-carcinoma sequence is well described in the medical literature. [2]
Three pathways to colon and rectal carcinoma have been described:
-
Adenomatous polyposis coli (APC) gene adenoma-carcinoma pathway
-
Hereditary nonpolyposis colorectal cancer (HNPCC) pathway
-
Ulcerative colitis dysplasia
The APC adenoma carcinoma pathway involves several genetic mutations, starting with inactivation of the APC gene, which allows unchecked cellular replication at the crypt surface. With the increase in cell division, further mutations occur, resulting in activation of the K-ras oncogene in the early stages and p53 mutations in later stages. These cumulative losses in tumor suppressor gene function prevent apoptosis and prolong the cell's lifespan indefinitely. If the APC mutation is inherited, it will result in familial adenomatous polyposis syndrome.
Histologically, adenomas are classified in three groups: tubular, tubulovillous, and villous adenomas. K-ras mutations and microsatellite instability have been identified in hyperplastic polyps. Therefore, hyperplastic polyps may also have malignant potential in varying degrees. [9]
The other common carcinogenic pathway involves mutation in DNA mismatch repair genes. Many of these mismatched repair genes have been identified, including hMLH1, hMSH2, hPMS1, hPMS2, and hMSH6. Mutation in mismatched repair genes negatively affects the DNA repair. This replication error is found in approximately 90% of HNPCC and 15% of sporadic colon and rectal cancers. [2, 10] A separate carcinogenic pathway is also described in inflammatory bowel disease (IBD). Chronic inflammation such as in ulcerative colitis can result in genetic alterations that lead to dysplasia and carcinoma formation. [2]
Etiology
Colorectal cancer appears to be multifactorial in origin and includes environmental factors and a genetic component. Approximately 75% of colorectal cancers are sporadic and develop in people with no specific risk factors. The remaining 25% of cases occur in people with significant risk factors—most commonly, a family history or personal history of colorectal cancer or polyps, which are present in 15-20% of all cases. Other significant risk factors are certain genetic predispositions, such as hereditary nonpolyposis colorectal cancer (HNPCC; 4-7% of all cases) and familial adenomatous polyposis (FAP, 1%); and inflammatory bowel disease (IBD; 1% of all cases).
Diet
A cohort study by Tabung et al that followed 121,050 adults for 26 years found that in both men and women, intake of proinflammatory diets (replete in red, processed, and organ meat, for example) was associated with a significantly higher risk of developing colorectal cancer. Risk was especially high in overweight and obese men and, paradoxically, in lean women. Risk was also increased in men and women who do not drink alcohol. [11]
A high-fat, low-fiber diet is implicated in the development of colorectal cancer. Specifically, people who ingest a diet high in saturated animal fats and highly saturated vegetable oils (eg, corn, safflower) have a higher incidence of colorectal cancer. The mechanism by which these substances are related to the development of colorectal cancer is unknown.
Saturated fats from dairy products do not have the same carcinogenic effect, nor do oils containing oleic acid (eg, olive, coconut, fish oils). Omega-3 monounsaturated fatty acids and omega-6 monounsaturated fatty acids also appear to be less carcinogenic than unsaturated or polyunsaturated fats. In fact, epidemiologic data suggest that high fish consumption may provide a protective effect against development of colorectal cancer. Long-term diets high in red meat or processed meats appear to increase the risk of distal colon and rectal cancers. [12, 13]
The ingestion of a high-fiber diet may be protective against colorectal cancer. Fiber causes the formation of a soft, bulky stool that dilutes carcinogens; it also decreases colonic transit time, allowing less time for harmful substances to contact the mucosa. The decreased incidence of colorectal cancer in Africans is attributed to their high-fiber, low–animal-fat diet. This favorable statistic is reversed when African people adopt a western diet. Findings of a meta-analysis of 22 studies with a total of 2,876,136 subjects suggest that dietary fiber intake could be a protective factor against rectal cancer with a clinically relevant reduction in rectal cancer risk. [12]
Increased dietary intake of calcium appears to have a protective effect on colorectal mucosa by binding with bile acids and fatty acids. The resulting calcium salts may have antiproliferative effects, decreasing crypt cell production in the mucosa. A double-blind placebo-controlled study showed a statistically significant reduction in the incidence of metachronous colorectal adenomas. [14] Other dietary components, such as selenium, carotenoids, and vitamins A, C, and E, may have protective effects by scavenging free-oxygen radicals in the colon.
Alcohol
Alcohol intake of more than 30 g daily has been associated with increased risk of developing colorectal carcinoma, with risk of rectal cancer greater than that of colon cancer. Risk appears greater with beer than with wine. [15] Specifically, Kabat et al found that daily beer consumption of 32 ounces or more increases the risk of rectal cancer in men (odds ratio 3.5). [16]
Tobacco
Smoking, particularly when started at a young age, increases the risk of colorectal cancer. [17] Possible mechanisms for tumor development include the production of toxic polycyclic aromatic amines and the induction of angiogenic mechanisms due to tobacco smoke.
A study by Phipps et al found that smoking is also associated with increased mortality after colorectal cancer diagnosis, especially among patients with colorectal cancer with high microsatellite instability. [18]
Cholecystectomy
Following cholecystectomy, bile acids flow freely, increasing exposure to the degrading action of intestinal bacteria. This constant exposure increases the proportion of carcinogenic bile acid byproducts. A meta-analysis by Giovannucci et al revealed an increased risk of proximal colon carcinoma following cholecystectomy. Although a large number of studies suggest the increased risk of proximal colon cancer in patients following cholecystectomy, the data are not compelling enough to warrant enhanced screening in this patient population. [2]
Family and personal history
The relative risk of developing colorectal cancer is increased in the first-degree relatives of affected patients. For offspring, the relative risk is 2.42 (95% confidence interval [CI]: 2.20-2.65); when more than one family member is affected, the relative risk increases to 4.25 (95% CI; 3.01-6.08). If the first-degree family member is younger than 45 years at the time of diagnosis, the risk increase is even higher. [19]
Regarding the personal history of colorectal cancer or polyps: Of patients with colorectal cancer, 30% have synchronous lesions, usually adenomatous polyps. Approximately 40-50% of patients have polyps on a follow-up colonoscopy. Of all patients who have adenomatous polyps discovered on colonoscopy, 29% of them have additional polyps discovered on a repeat colonoscopy one year later. Malignancy develops in 2-5% of patients. The risk of cancer in people who have had polyps removed is 2.7-7.7 times that of the general population. [20]
Familial adenomatous polyposis (FAP)
FAP is an autosomal dominant inherited syndrome that results in the development of more than 100 adenomatous polyps and a variety of extra-intestinal manifestations. The defect is in the APC gene, which is located on chromosome 5 at locus q21. The disease process causes the formation of hundreds of intestinal polyps, osteomas of bone, desmoid tumors, and, occasionally, brain tumors. Individually, these polyps are no more likely to undergo malignant transformation than are polyps in the general population. The increased number of polyps, however, predisposes patients to a greater risk of cancer. If left untreated, colorectal cancer develops in nearly 100% of these patients by age 40. Whenever the hereditary link is documented, approximately 20% of FAP cases are found to be caused by spontaneous mutation.
Hereditary nonpolyposis colorectal cancer
HNPCC, or Lynch syndrome, is an autosomal dominant inherited syndrome that occurs because of defective mismatch repair genes located on chromosomes 2, 3, and 7. Patients have the same number of polyps as the general population, but their polyps are more likely to become malignant. These patients also have a higher incidence of endometrial, gastric, thyroid, and brain cancers.
The revised Amsterdam criteria are used to select at-risk patients (all criteria must apply):
-
Three or more relatives who are diagnosed with an HNPCC-associated cancer (colorectal, endometrium, small bowel, ureter, or renal pelvis)
-
One affected person is a first-degree relative of the other two
-
One or more cases of cancer are diagnosed before age 50 years
-
At least two generations are affected
-
FAP has been excluded
-
Tumors have undergone a pathology review
The National Comprehensive Cancer Network (NCCN) guidelines discuss various strategies for identifying patients who should undergo testing for HNPCC. One approach is to test patients with any of the following [21] :
-
Personal history of colorectal, endometrial, or other Lynch syndrome–associated cancer
-
Suspicious family history
-
≥5% risk of having a mismatch repair (MMR) gene pathogenic variant, based on predictive models (PREMM5,11 MMRpro, MMRpredict)
Another strategy is so-called universal screening, in which all individuals newly diagnosed with colorectal cancer undergo either microsatellite instability (MSI) or immunohistochemistry (IHC) tumor testing for absence of 1 of the 4 DNA MMR proteins. An alternative is to test all patients with colorectal cancer diagnosed before age 70 years, and test those 70 years and older only if they meet the Bethesda criteria for colorectal cancer. The primary method for detecting HNPCC in tumor tissue from biopsied or surgically resected specimens is with either immunohistochemistry or microsatellite instability testing. The NCCN guidelines also indicate that genetic counseling is not necessary before “routine tumor testing” at a center. [21]
Inflammatory bowel disease
The malignant pathway in individuals with inflammatory bowel disease does not involve any adenoma-carcinoma sequence. Cancer risk increases with duration of disease. After 10 years, the incidence of colorectal cancer in ulcerative colitis (UC) is approximately 1% per year. Patients should be evaluated for dysplastic changes via an annual colonoscopy. Dysplasia is a precursor of cancer and when present, the risk of cancer is 30%.
The incidence of colorectal cancer in patients with Crohn disease is 4-20 times greater than that of the general population. Cancer occurs in patients with disease of at least 10 years' duration. The average age at cancer diagnosis, 46-55 years, is younger than that of the general population. Cancers often develop in areas of strictures and in de-functionalized segments of intestine. In patients with perianal Crohn disease, malignancy is often present in fistulous tracts. Patients with Crohn colitis should undergo the same surveillance regimen as those with UC.
Epidemiology
United States
Colon and rectal cancer is the third most common cancer in both females and males. The American Cancer Society (ACS) estimates that 107,320 new cases of colon cancer and 46,950 new cases of rectal cancer will occur in 2025—27,950 cases of rectal cancer in men and 19,000 in women. For estimates of deaths, the ACS combines colon and rectal cancers, because many deaths from rectal cancer are misclassified as due to colon cancer on death certificates; approximately 52,900 deaths from colorectal cancer are expected to occur in 2025. [1]
The incidence of colorectal cancer has generally declined since the mid-1980s. The decrease has accelerated since 2000, thanks largely to greater use of screening. However, the overall trend is driven by older adults (who have the highest rates) and masks the situation in younger adults, who have experienced rising incidence rates since at least the mid-1990s. From 2012 to 2021, incidence rates decreased by about 1% per year overall, but rates in persons younger than 50 years increased by 2.4% per year and rates in those age 50-64 increased by 0.4% per year. [1] Currently, adults born circa 1990 have quadruple the risk of rectal cancer compared with those born circa 1950. [22]
The overall death rate from colorectal cancer has also been falling, decreasing 57% from 1970 to 2022—from 29.2 to 12.6 per 100,000, respectively—because of changing patterns in risk factors, increased screening, and improvements in treatment. During the past decade, the death rate declined by 1.7% per year. As with incidence rates, however, the decrease in overall mortality masks the rise in death rates in adults younger than 55 years, which have increased by about 1% per year since the mid-2000s. [1]
Tumor site tends to vary by patient age. In those aged younger than 65 years, the rectum is the most common site of colorectal cancer, accounting for 42% of cases in those age 20 to 49 years and 38% in those 50 to 64 years of age. In individuals age 65 and older, rectal cancer accounts for 24% of colorectal cancer cases; the proximal colon is the most common colorectal cancer site in that age group, accounting for 47% of cases. [23]
International
An estimated 729,833 new cases of rectal cancer occurred worldwide in 2022, making it the eighth most common cancer. Incidences varied considerably by country, with high rates in Europe and Australia–New Zealand, and low rates in western and middle Africa and south-central Asia. [24]
Race
The incidence of colorectal cancer tends to be higher in Western nations than in Asian and African countries; however, within the United States, differences in incidence exist among Whites, Blacks, and Asians: the rate of new cases per 100,000 population is highest in Blacks (52.4 in men, 38.6 in women), then Whites (43.5 in men, 33.3 in women), then Hispanics (41.1 in men, 29.0 in women). In the US, Black men have the highest mortality (22.3 per 100,000 population) and Asian/Pacific Islander women and Hispanic women have the lowest mortality (7.7 and 8.5 per 100,000 population, respectively). [25] However, the incidence of colorectal cancer among people younger than 50 years is increasing much faster in Whites than in Blacks (2% vs 0.2% per year, respectively). [23]
A study by Yothers et al found that Black patients with resected stage II and stage III colon cancer had worse overall and recurrence-free survival compared with White patients who underwent the same therapy. [26]
A study of racial disparities in mortality rates between Black and White individuals with colorectal cancer by Robbins et al showed earlier and larger reductions in death rates for Whites from 1985-2008. [27] This racial disparity could be decreased with greater education to the Black population regarding colorectal cancer prevention and access to treatment, including colonoscopies and polypectomies.
Sex
The incidence of colorectal malignancy is slightly higher in males than in females. The overall age-adjusted incidence of colorectal cancer in all races was 43.4 per 100,000 for males and 32.8 per 100,000 for females in 2015-2019, yielding a male-female ratio of 1.32:1. Mortality rates for colorectal cancer in 2016-2020 were also higher in males (15.7 per 100,000) than in females (11.0 per 100,000). [25]
Age
The incidence of colorectal cancer starts to increase after age 35 and rises rapidly after age 50, peaking in the seventh decade. More than 90% of colon cancers occur after age 50. However, cases have been reported in young children and adolescents. [2] In the United States, the incidence rates of colorectal cancer increased by more than 2% per year in adults younger than age 50 from 2012 to 2021, largely because of increases in rectal cancer. [1]
Incidence rates of colorectal cancer in persons younger than 50 years have also increased in many other high‐income countries aside from the US, including Australia, Canada, Germany, and the United Kingdom. In Austria, however, where opportunistic screening has been used in individuals aged 40 years and older since the 1980s, rates of colorectal cancer are increasing in those aged 20 to 39 years but decreasing in those age 40 to 49 years. [23]
A study by Sung et al that examined colorectal cancer incidence trends in younger adults versus older adults in 50 countries and territories found that from 2013 to 2017, early-onset colorectal cancer (diagnosed at ages 25 to 49 years) increased in 27 countries. The greatest annual increases occurred in New Zealand (3.97%), Chile (3.96%), Puerto Rico (3.81%), and England (3.59%). In 14 of those 27 countries and territories, rates in older adults were either stable or decreased. [28]
Mortality/morbidity
The American Cancer Society estimates that in 2025, colorectal cancer will account for 9% of cancer deaths in men and 8% in women, making it the third and fourth most common cause of cancer deaths, respectively. In the US, mortality rates have been decreasing in both sexes for the past 2 decades. The 5-year relative survival rate is 65% for colorectal cancer; however, for patients who are diagnosed with localized disease, the 5-year survival rate is 91.1%. [25]
Prognosis
The 5-year relative survival rates for colorectal cancer based on SEER stage are as follows [25] :
-
Localized - 91.1%
-
Regional - 73.7%
-
Distant - 15.7%
-
All stages - 65.0%
A review of 111,453 patients in the National Cancer Data Base who were diagnosed with early-stage (T1N0 or T2N0) rectal cancer from 1998 to 2010 found that increasing age, male sex, higher comorbidity score, and positive or unknown final surgical margins were associated with poorer long-term adjusted overall survival. [29]
Recurrence of rectal cancer, which usually develops in the first year after surgery, carries a poor prognosis. Recurrence may be local, distant, or both; local recurrence is more common in rectal cancer than in colon cancer. Reported rates of local recurrence have ranged from 3.7% to 50%. [30] Factors that influence the development of recurrence include the following:
-
Surgeon variability
-
Grade and stage of the primary tumor
-
Location of the primary tumor (eg, low rectal cancers have higher rates of recurrence
-
Ability to obtain negative margins
Surgical therapy may be attempted for recurrence and includes pelvic exenteration or abdominal perineal resection in patients who had a sphincter-sparing procedure. Radiation therapy generally is used as palliative treatment in patients who have locally unresectable disease.
Patient Education
A study by Thong et al found that survivors of rectal cancer may benefit from increased focus, both clinical and psychological, on the possible long-term morbidity of treatment and its effects on health. [31] Survivors should also receive education on the possible benefits of secondary prevention with dietary changes (see Treatment/Diet) and aspirin (see Treatment/Prevention).
-
Diagnostics. Staging and workup of rectal cancer patients.
-
Staging and treatment. Rectal cancer treatment algorithm (surgery followed by adjuvant chemotherapy and radiotherapy). Initial stages are Endorectal ultrasound staging (uT).