Overview
Cognitive disorder in the setting of human immunodeficiency virus (HIV) infection has long been recognized as an important and disabling aspect of the disease. It has been known by a variety of terms. The current terminology, HIV-associated neurocognitive disorder (HAND) and its subclassifications, were articulated at the Frascati conference in 2004. [1] In addition to its effects on the cellular immune system, HIV enters the central nervous system (CNS) early in the course of the infection and causes several important CNS conditions over the course of the disease, such as HIV encephalopathy and HAND. As part of the acute HIV syndrome during seroconversion, patients may experience HIV encephalopathy. HIV-associated progressive encephalopathy (HPE) is a syndrome complex with cognitive, motor, and behavioral features seen in children. [2]
Prior to the advent of highly active antiretroviral therapy (HAART), dementia was a common source of morbidity and mortality in HIV-infected patients. It was usually observed in the late stages of acquired immunodeficiency syndrome (AIDS), when CD4+ lymphocyte counts fall below 200 cells/mL, and was seen in up to 50% of patients prior to their deaths. In 1986, the term HIV-associated dementia (HAD) was introduced to describe a unique constellation of neurobehavioral findings. [3] As HIV treatment entered the HAART era, neurocognitive dysfunction has remained prevalent, with cross-sectional studies suggesting some degree of cognitive impairment in 44% of patients. [4] The nature and severity of the impairment changed with the advent of prevalent HAART therapy, however, leading to the new consensus definition of HIV-associated neurocognitive disorder in 2004. [5]
HAND encompasses a hierarchy of progressively more severe patterns of neurologic involvement. It is defined as an acquired impairment in cognitive functioning involving at least 2 ability domains with a decline of daily functioning in severe cases. The spectrum ranges from asymptomatic neurocognitive impairment (ANI) to minor neurocognitive disorder (MND) to more severe HIV-associated dementia (HAD). [1, 5]
While in the pre-HAART era, HAD developed predominantly in patients with high viral loads and CD4 counts < 200, whereas HAND develops in patients with normal CD4 counts. [4]
Forno et al found that among 67 HIV-positive patients, 18 were diagnosed with asymptomatic neurocognitive impairment (ANI) and 21 with mild neurocognitive disorder (MND), whereas 28 showed no cognitive deficits. [6] There was a significant correlation between neurological soft signs (NSS) scores and various cognitive domains, suggesting that NSS are closely associated with neuropsychological deficits similar to those seen in schizophrenia. This indicates that NSS examinations could be useful in screening for HIV-associated neurocognitive disorders (HAND), demonstrating their broad relevance across different severe mental disorders.
The overall psychosocial and emotional burden on the family and friends of patients with HAND remains tremendous. Patients with cognitive difficulties have problems with adherence to their medication regimens, follow-up visits, and health behavior. Because of their neuropsychiatric problems, such patients may be more prone to risk behavior (eg, unprotected intercourse), and they may pose a greater risk for transmission of the virus.
In addition to HIV itself, other causes of neurologic complications in HIV-infected individuals include opportunistic infections, tumors, autoimmune conditions, and antiretroviral drugs. In addition, patients are at risk for many other neurologic complications of HIV infection, including vacuolar myelopathy, peripheral neuropathies, and polymyositis, which can contribute to further disability.
For other discussions of HIV infection, see HIV Disease, Pediatric HIV Infection, and Antiretroviral Therapy for HIV Infection.
Pathophysiology
Cellular proteins
HIV enters the brain primarily through infected macrophages and lymphocytes, with other potential entry mechanisms including the passage of cell-free virus and release from infected endothelial cells. [7, 8] Once inside the CNS, the virus replicates in various cell types including microglia, oligodendrocytes, astrocytes, and neurons. The most commonly infected cells are macrophages and microglia. The viral presence and progression in the CNS can be monitored by cerebrospinal fluid (CSF) viral load measurements, which show a positive correlation with the extent of cognitive dysfunction.
Pathologic changes observed at autopsy are predominantly subcortical, involving deep gray matter such as the basal ganglia and thalamus, as well as white matter regions. [7] The mechanism by which HIV infection leads to neurocognitive disorders is multifactorial, involving indirect cellular responses. These include the secretion of chemokines, proinflammatory cytokines, nitrous oxide, and other neurotoxic factors from both infected cells (ie, macrophages, astrocytes, microglia) and noninfected activated cells. Chemokines such as CCL4 and CXCL12 and their receptors, CCR5 and CXCR4, are particularly noteworthy as they influence many cellular processes including neuronal migration, apoptosis, and neurotransmitter regulation.
A meta-analysis found that patients with HIV-associated neurocognitive disorders (HAND) show significant alterations in white matter integrity compared to healthy controls, as evidenced by lower fractional anisotropy and higher mean diffusivity in DTI measurements. [9] Despite substantial heterogeneity among studies due to factors like demographics and antiretroviral therapy duration, subgroup analysis significantly reduced this variability. Although DTI is valuable for diagnosing HAND, its standalone diagnostic use is limited without neurocognitive function scales.
HIV proteins (virotoxins)
Studies in transgenic mouse models have shown that the expression of single or multiple HIV genes leads to clinical and histologic abnormalities. [7] Specific HIV proteins such as gp120, gp41, Tat, Nef, Vpr, and Rev may be directly toxic to neuronal cells or may cause damage indirectly by activating astrocytes, microglia, and macrophages to release cytokines, chemokines, or neurotoxic substances. The basal ganglia show the highest immunostaining for the HIV p24 antigen, and the expression of gp41 in the basal ganglia and frontal lobes significantly correlates with dementia severity. However, the presence of macrophages and microglia correlates better with clinical dementia than the density of HIV-infected cells in the brain.
Moreover, HIV proteins Tat and gp120 cause microglia to release factors that promote neuronal p53 activation, leading to cell cycle arrest and apoptosis in neurons through oxidative injury and DNA damage. [7] The synergistic neurotoxicity of Tat and gp120 with substances such as cocaine and methamphetamine further increases the risk of developing AIDS dementia complex (ADC), highlighting the complex interactions between HIV infection, host cellular responses, and external factors. [3, 10, 11, 12]
The prevalence of HIV-associated neurocognitive disorders (HAND) remains high, estimated to occur in 30% to 60% of patients, with variations across different regions. Studies like the CHARTER cohort have reported a prevalence of HAND at 47%, indicating significant clinical implications and underscoring the need for enhanced diagnostic and therapeutic strategies. [4] This complex interplay of factors in the pathogenesis of HAND necessitates a comprehensive understanding and approach to managing this condition in people living with HIV.
Autoimmune disease
CNS damage may occur by humoral immune mechanisms, as evidenced by the presence of anti-CNS antibodies in AIDS patients with dementia but not in those without dementia. [13] Autoantibodies against myelin oligodendrocyte glycoprotein may persist in a high percentage of patients despite viral clearance; this finding suggests ongoing neuroinflammation, which may prevent recovery from HIV-associated neurocognitive disorder. [14]
Risk factors
In the pre-HAART era, the identified risk factors for HAD were low weight, anemia, constitutional symptoms, low CD4+ count, and high plasma HIV-RNA load. In the post-HAART era, the most significant risk factor for HAND is CD4 nadir, but risk factors also include age, hypertension, and hyperlipidemia. [15] This identification, along with the neuroimaging similarities between HAND and vascular cognitive impairment, has led some to postulate that premature aging at the neurovascular unit may be the primary target of HIV-related brain injury in the treated HIV population. [16]
The Multicenter AIDS Cohort Study reported that older age was associated with more rapid progression to dementia and death. [17] The prevalence of cognitive disorders in persons who are HIV positive and older than 50 years is greater than in younger patients. [18]
Epidemiology
The prevalence of HIV-associated neurocognitive disorders (HAND) has remained high over the past two decades, affecting 30% to 60% of patients, with no significant changes noted. [19] HAND prevalence is notably higher in Latin America and the Caribbean. The CHARTER study, a large cohort analysis, reported a 47% prevalence of HAND, breaking down to 33% with asymptomatic neurocognitive impairment (ANI), 12% with mild neurocognitive disorder (MND), and 2% with HIV-associated dementia (HAD). [20] However, the CHARTER cohort might not fully represent the broader HIV population owing to potential sample biases and other limitations such as the lack of demographically adjusted norms and the possibility of mode effects from using patient self-reports and clinician ratings. The data, collected between 2003 and 2007, might not accurately reflect the current state of cognitive impairment in people living with HIV (PLWH). Additionally, a meta-analysis showed that HAND subtypes' prevalence was 23.5% for ANI, 13.3% for MND, and 5% for HAD, independent of CD4 count, ART status, or Hepatitis C virus (HCV) co-infection.
A meta-analysis attempting to quantify the global prevalence of HAND estimated it to be 42.6% with 88% of cases representing mild disease. The proportion of patients with HAND decreased with level of income, CD4 count, and treatment with HAART. Findings suggest that early initiation of HAART and prevention of severe immunosuppression (and, thus, CD4 nadir) reduced both the prevalence and the severity of HAND. The prevalence in sub-Saharan Africa and Latin America may be higher than in the United States and Western Europe. [21]
Prognosis
The prognosis of HIV-associated neurocognitive disorder (HAND) is dependent on its severity. Little is known about the expected progression of asymptomatic neurocognitive impairment (ANI) and minor neurocognitive disorder (MND), but extrapolating from the Multicenter AIDS Cohort Study, which found that patients with HIV infection had no more decline in neuropsychometric tests than age-matched controls, suggests that cognitive deterioration in HAND patients is not widespread. The CHARTER study, similarly, found that most patients (61%) remained stable over a 42-month period whereas 16% improved and 22% declined. Patients who experienced progression were more likely to have severe comorbidities such as drug use and hepatitis C as well as a higher rate of HAART treatment failure and lower CD4 count. [1, 20]
HIV-associated dementia (HAD) is associated with a much worse prognosis, and typically is associated with progressive impairment of attention and concentration, motor slowing, and behavioral change with death within 1 year of presentation. Patients who are severely impacted and meet criteria for HAD based on neuropsychological evaluation but do not show evidence of progression are suspected of having a better prognosis.
There is a critical need for longitudinal cohort studies and multimodal neuroimaging to enhance the understanding of the clinical prognosis and neurocognitive mechanisms of HIV-associated neurocognitive disorder (HAND), as well as an urgency of establishing a standardized diagnostic process for HAND to improve clinical outcomes and the quality of life for those affected. [22]
A meta-analysis found the estimated prevalences of HAND, asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) were 44.9%, 26.2%, 8.5%, and 2.1%, respectively. [22] Factors found to be associated with HAND included the percentage of females in the study, current CD4 count, education level, and the development level of the country, all showing significant correlations.
Patient Education
Educate the patient at an early stage, and discuss future medicolegal implications of dementia. The patient should be strongly encouraged to prepare a living will and to assign power of attorney.
Educate patients and families about the persistent dangers of the transmission of HIV. The following Web site can be referenced for further information:
-
UCSF Center for HIV Information – HIV Transmission and Prevention in Adolescents
Education of family members, friends, and caregivers of a patient with HIV-associated neurocognitive disorder (HAND) is of great importance. HIV dementia is a multifaceted problem, and caregivers should know about the complications, including psychiatric complications. Often, friends and family need counseling and support to deal with this chronic and difficult condition.
For patient education information, see HIV Infection and AIDS.
Clinical Presentation
It is estimated that nearly half of all patients with HIV infection demonstrate lower than expected performance in various neuropsychological assessments compared with healthy individuals matched for age, race, education, and ethnicity. Neuropsychiatric symptoms such as apathy, depression, anxiety, and psychosis are commonly seen in these individuals. [23] Significant memory impairment is also seen in these individuals. Personality changes, including social withdrawal and, in some extreme cases, vegetative state and mutism, are also seen.
Not all patients with HIV-associated neurocognitive disorder (HAND) progress to HIV-associated dementia (HAD). The onset of HAD is correlated with high viral loads, CD4 nadir, and the duration of the infection itself. With the use of HAART, milder forms of cognitive dysfunction, asymptomatic neurocognitive impairment (ANI) and minor neurocognitive disorder (MND), have become common.
Cognitive assessment in patients with HAD demonstrates deficits in memory, abstraction, verbal fluency, decision-making, and attention.
Rare features include sleep disturbances, psychosis (with mania), and seizures. Motor problems include imbalance, clumsiness, and weakness.
Early signs and symptoms are subtle and may be overlooked. Consequently, cognitive screening tests should be part of the routine care of HIV-infected patients, especially those at high risk, as indicated by older age, high plasma HIV ribonucleic acid (RNA) levels, low CD4+ cell counts, hepatitis C, and poor baseline cognitive status.
In ANI and MND, activities of daily living are only mildly impaired. This contrasts with the significant impairment seen in HAD.
In infants with HIV-associated progressive encephalopathy (HPE), characteristic features include decline in intellectual and motor milestones. In young children, the rate of acquisition of new skills decreases, and fine motor ability and dexterity may become impaired. Feeding difficulties may develop. In older children and adolescents, the presentation is similar to that of AIDS dementa complex (ADC) in adults.
Physical Examination
The examination for AIDS dementa complex (ADC) includes a full mental status examination (MSE), a general neurologic examination, and a general physical examination. The patient should then be referred for complete neuropsychological testing addressing specific domains.
Some patients with HIV can become depressed, suicidal, and homicidal. Therefore, a thorough psychiatric assessment should also be performed at each visit.
In the early stage, findings from the MSE and the general neurologic examination are normal. MSE findings are abnormal if the patient exhibits inattention, impaired concentration (ie, digit span, serial 7's), memory loss (ie, recalling 3 objects at 5 min), slowed verbal responses, and a blunted affect.
Early on, the neurologic examination may be normal or may reveal subtle impairment of rapid limb and eye movements. In more severe cases, the neurologic examination shows frontal release signs, slowed rapid movements, antisaccadic eye movements, incoordination, abnormal gait, hyperreflexia, hypertonia, extensor-plantar response weakness, and peripheral neuropathy. [10] Cortical signs, including apraxia, aphasia, and agnosia, typically are absent.
The terminal stage of HIV-associated dementia (HAD), after progression over several months, includes severe psychomotor retardation and dementia, apraxia, paraparesis, and akinetic mutism. Death ensues within a few months of reaching this stage.
Seizures are rare and warrant consideration of other conditions.
Diagnostic Criteria
AIDS dementia complex (ADC) encompasses cognitive deficits, behavioral changes, and motor involvement. Affected persons may manifest deficits in each of the 3 aspects at varying severity; thus, some patients may present primarily with cognitive changes, such as slowed processing of information, captured by neuropsychological testing; others may present with behavioral problems; and still others may be have a chief complaint of motor symptoms, such as unsteady gait, tremor, or weakness.
In 1991, the American Academy of Neurology defined neurocognitive deficits seen with HIV as HIV-associated dementia (HAD) and minor cognitive motor disorder (MCMD). Criteria for the diagnosis of HAD included cognitive deficits in 2 or more cognitive domains that cause impairment in activities of daily living (ADL) and an abnormality in either motor or neurobehavioral function. Patients with MCMD were defined as having decreased function in 2 cognitive or behavioral domains but without severe enough impairment to meet criteria for HAD.
In 2007, Antinori et al proposed more refined criteria for diagnosing cognitive impairment associated with HIV. Using the Frascati criteria, a recognized classification system, they proposed 3 entities: asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). This system evaluates the extent and type of cognitive deficits, the level of functional impairment, and rules out other potential causes of cognitive decline. [23]
Standardized neuropsychological testing was required to assess the following domains of cognition: language, attention, executive function, memory, speed of information processing, and perceptual and motor skills. In order to meet diagnostic criteria for ANI, MND, or HAD, patients must have no other etiology of dementia and must not have the confounding effect of substance use or psychiatric illness. [5]
ANI was proposed on the basis of experience with patients who had declines in their performance on formal neuropsychological testing but had no functional impairment in ADL. One standard deviation (SD) below the mean for age and education appropriate norms in at least 2 of at least 5 tested domains in formal testing is diagnostic of ANI.
Patients with MND meet the criteria for ANI, except that they also have impairment with ADL as reported by patient or by a corroborator. HAD can be diagnosed if patients score 2 SDs below the mean in at least 2 cognitive domains and have marked impairment in ADL as a result of cognitive decline.
Staging of AIDS Dementia Complex
Price and Brew in 1988 outlined a clinical staging of AIDS dementia complex (ADC), the Memorial Sloan-Kettering rating scale. [11] It is used mostly as a research instrument.
Stage 0
In stage 0 (normal), mental and motor functions are normal. In stage 0.5 (equivocal/subclinical), symptoms may be absent, minimal, or equivocal, with no impairment of work or performance of ADL. Mild signs (snout response, slowed ocular or extremity movements) may be present, but gait and strength are normal.
Stage 1
In stage 1 (mild), the patient is able to perform all but the more demanding aspects of work or activities of daily living (ADL) but has unequivocal evidence of functional, intellectual, or motor impairment. Signs or symptoms may include diminished performance on neuropsychological testing. Patient can walk without assistance.
Stage 2
In stage 2 (moderate), the patient is able to perform basic activities of self-care but cannot work or maintain the more demanding aspects of daily life. The patient is ambulatory but may require a single prop.
Stage 3
In stage 3 (severe), the patient has major intellectual incapacity (cannot follow news or personal events, cannot sustain complex conversation, shows considerable slowing of all outputs). Motor disability precludes walking unassisted (ie, without walker or personal support); walking is usually slowed and accompanied by clumsiness of arms.
Stage 4
In stage 4 (end stage), the patient is in a nearly vegetative state. Intellectual and social comprehension and output are at a rudimentary level. The patient is nearly or absolutely mute. The patient is paraparetic or paraplegic, with urinary and fecal incontinence.
Neurologic Examination in Pediatric Patients
Despite the availability of antiretroviral treatment (ART), cognitive impairments and learning difficulties are still common in HIV-infected children. [24] These challenges are linked to several biological factors, including persistent HIV reservoirs in the brain, CNS inflammation, disrupted neuronal function, and the infiltration of activated HIV target cells during brain development. Developing treatments that target these mechanisms, such as chemokine receptor antagonists, is critical. Additionally, employing psychosocial strategies that capitalize on neuroplasticity to support brain development is essential. A holistic approach that integrates these strategies is key to enhancing neurodevelopmental outcomes. Dynamic neuropsychological assessments, such as computerized cognitive games, along with monitoring neuroinflammatory biomarkers and brain-derived neurotrophic factors (BDNFs), are advised to assess the efficacy of new treatments and the overall brain and behavioral health of these children.
In neonates, physical examination findings typically appear normal. Symptoms usually begin in the first year of life, although they may not become apparent until ages 2 to 3 years, when children might exhibit cognitive impairment, masklike facies, acquired microcephaly, and signs of pseudobulbar and corticospinal tract involvement.
In older children and adolescents, common symptoms include impaired attention, reduced linguistic and academic performance, psychomotor slowing, emotional instability, and social withdrawal. These examination findings are similar to those observed in adults with AIDS dementia complex (ADC).
Criteria for HIV-associated progressive encephalopathy
The American Academy of Neurology defines HIV-associated progressive encephalopathy (HPE) as the presence, for at least 2 months, of at least one of the following progressive findings in a pediatric patient with no concurrent illness, other than HIV infection, that could explain the findings: [25]
-
Failure to attain, or loss of, developmental milestones or loss of intellectual ability verified by standard developmental or neuropsychological tests
-
Acquired microcephaly as demonstrated by head circumference measurement or brain atrophy on serial computed tomography (CT) or magnetic resonance imaging (MRI) imaging in children younger than 2 years of age
-
Acquired symmetrical motor deficits manifested by 2 or more of the following: paresis, pathological reflexes, ataxia or gait disturbance
Differential Diagnosis
Dementia due to HIV disease is a diagnosis of exclusion. The differential diagnosis includes the following:
Other problems to be considered in the differential diagnosis include the following:
-
Cerebral lymphoma
-
CNS infections (eg, tuberculosis, toxoplasmosis, cryptococcal meningitis, neurosyphilis)
-
Metabolic encephalopathies
-
Depression
-
Psychoactive drugs
-
Attention deficit hyperactivity disorder
-
Toxic-metabolic states (eg, alcoholism, vitamin B-12 deficiency, thyroid disorders, adverse medication effects, drug interaction, recreational drug use)
Antiretroviral medications may have neurocognitive side effects (eg, efavirenz therapy can result in depression, insomnia, and decreased neuropsychological testing). [26]
Immune reconstitution inflammatory syndrome (IRIS)
Clinical worsening may be observed in patients with HIV soon after initiation of HAART therapy, due to mounting of a significant inflammatory response. This is seen even while a patient’s CD4+ count improves and viral load dramatically decreases. Immune reconstitution inflammatory syndrome (IRIS) may actually worsen AIDS dementia complex (ADC) and progressive multifocal leukoencephalopathy. [27]
ADC can be distinguished from IRIS by the speed of onset. ADC is subacute to chronic, whereas IRIS can be more acute to subacute. ADC occurs in the setting of untreated, progressive AIDS, whereas IRIS begins with the start of treatment. Distinguishing between the 2 is important, because the treatment is different. ADC requires HAART with high penetration into the CNS. IRIS should be treated with steroids, depending on its severity.
An entity termed encephalitis with CD8 cell infiltration has been identified in patients with latent or inactive HIV infection of the brain due to restoration of T-cell function with HAART, leading to an intense inflammatory reaction with an influx of CD8+ lymphocytes. The influx of the CD8 cells may cause macrophage activation and control of infection but can also present with a subacute or acute encephalopathy. The imbalance between CD8+/CD4+ cells may cause worsening of the HIV encephalopathy and can also produce an acute demyelinating process similar to multiple sclerosis or acute demyelinating encephalomyelitis.
These cases may represent a specific clinicopathological entity, of which a few comparable cases have already been described. They can be included in the wide framework of immune reconstitution disease. Such syndromes have been described with opportunistic infections, but only rarely with HIV infection of the CNS. [28] In a report of 14 patients treated with glucocorticosteroids, therapeutic response varied from excellent, with no sequelae (n=5), to moderate, with cognitive disorders (n=4). The mean survival time was 8 years; however, 5 patients died within 13 months of the initiation of treatment.
Overview of Workup
The workup includes lumbar puncture with cerebrospinal fluid (CSF) analysis, neuroimaging, and neuropsychological testing. MRI is the first-choice neuroimaging modality. Electroencephalography (EEG) reveals generalized slowing in the later stages; this is a nonspecific finding. Brain biopsy is not recommended for corroboration, but biopsy material obtained for other reasons may confirm this diagnosis.
Laboratory tests are essential in the diagnostic process and include a complete blood count, liver and renal function tests, as well as assessments of vitamin B12 and folate levels, syphilis serology, and thyroid function. [23] These investigations are conducted to detect any underlying health issues that might be contributing to cognitive impairments.
Draw peripheral blood for syphilis serology testing, thyroid studies, routine electrolyte levels, blood urea nitrogen (BUN)/creatinine determination, and a drug screen to effectively exclude other metabolic and infectious etiologies.
Vitamin B-12 and folic acid levels should be determined and, if necessary, corrected (see Vitamin B-12 Associated Neurological Diseases). In cases of borderline low B-12 levels, homocysteine and methylmalonic acid levels are better indicators of a deficiency. An assay for anti-parietal cell or anti-intrinsic factor antibodies and a Schilling test may be indicated.
Challenges in researching HIV-associated neurocognitive disorders (HAND) include diverse diagnostic criteria, limited availability of high-quality genetic material, and the frequent exclusion of HIV-negative controls. [29] These issues underscore the need for standardized neurocognitive assessments and improved methodologies in HAND research. Proposals for enhancing diagnostic accuracy include adjusting the scoring criteria for neurocognitive impairment and treating cognitive performance as a continuous variable, which could be retroactively applied to existing datasets for re-analysis.
Next-generation sequencing techniques like RNA-seq and whole exome sequencing (WES) have the potential to advance the understanding of HAND. [30] These methods have begun to uncover cell type-specific changes and broader genetic influences on HAND, although challenges remain, such as the quality of banked tissues and the scarcity of samples from cART-treated individuals. The National NeuroAIDS Tissue Consortium (NNTC) has been instrumental in providing resources for HAND research, but there is still a need for more comprehensive sample collections to enhance the statistical power of studies. Overall, integrating these advanced genomic tools and expanding available datasets are crucial for elucidating the complex genetic and pathological mechanisms underlying HAND.
Cerebrospinal Fluid Analysis
Cerebrospinal fluid (CSF) analysis is instrumental in assessing HIV-associated neurocognitive disorders (HAND). [23] Biomarkers in the CSF, such as neopterin, quinolinic acid, and β-2 microglobulin, can reveal signs of inflammation and immune activation in the central nervous system (CNS). Additionally, measuring HIV RNA levels in the CSF helps evaluate viral activity within the CNS. However, CSF analysis is generally conducted only in cases with ambiguous diagnoses or to rule out other CNS infections, as it is not a routine procedure.
CSF markers are helpful in early dementia, when the diagnosis may be confusing. These markers, including neopterin, quinolinic acid, certain cytokines (eg, tumor necrosis factor–alpha, interleukin 1, interleukin 6), and antibodies to gp120 (eg, HIV viral envelope protein), correlate with the severity of dementia, but they are only research tools and therefore are not widely available.
The axonal neurofilament light chain protein and neuronal protein Tau are frequently elevated in the CSF of patients with HIV-associated dementia (HAD). These proteins sometimes can be seen even before HAD develops and normalize with treatment. Neopterin, MCP-1, and CSF viral load can be used to assess the effect of treatment on viral replication.
CSF beta-2 microglobulin, an immune activation marker, is a more specific CSF marker and has a positive predictive value of 88% if levels are higher than 3.8 mg/dL. CSF beta-2 microglobulin levels were found to be twice as high in patients who cognitively improved with HAART than in those who did not, indicating that CNS inflammation plays a major role in reversible neurocognitive deficits. Patients with HIV dementia have elevated levels of certain matrix metalloproteins in the CSF, but the clinical significance of these proteins is unclear.
Beta-amyloid(1-42) measurements in CSF of patients with HIV who are cognitively impaired are similar to patients with mild dementia of the Alzheimer type (DAT). Normal or slightly depressed CSF tau and p-tau181 measurements distinguish patients with HIV-associated cognitive impairment from patients with DAT. [31]
Other CSF findings are as follows:
-
Elevated protein (60%) and immunoglobulin G (80%)
-
Oligoclonal bands are sometimes present
-
CSF usually is acellular, but a mononuclear pleocytosis is found in 25%
-
HIV antibodies are present
HIV frequently is cultured from CSF or detected by means of polymerase chain reaction (PCR). However, HIV-1 is present in CSF in the absence of neurologic abnormalities. CSF HIV RNA levels do not correlate with neuropsychological impairment; rather, plasma levels are a better correlation.
CSF studies to help exclude CNS infection include the following:
-
Cryptococcal antigen
-
Venereal Disease Research Laboratory (VDRL) test
-
Fluorescent treponemal antibody-absorption test (FTA-abs)
-
Cytomegalovirus polymerase chain reaction (PCR) test
Depending on the clinical picture, PCR studies may also be obtained for herpes simplex and varicella zoster viruses and JC virus (the causative agent of progressive multifocal leukoencephalopathy).
Neuroimaging
CT and MRI of the brain can support a diagnosis of HIV-associated neurocognitive disorder (HAND) and rule out other neurologic opportunistic infections or neoplasms. CT can reveal diffuse cortical atrophy, ventricular enlargement, and increased white matter signal in later stages (see the image below). Basal ganglia calcifications are seen in adults but are more common in children. Neuroimaging results may be normal in minor cognitive motor disorder (MCMD).
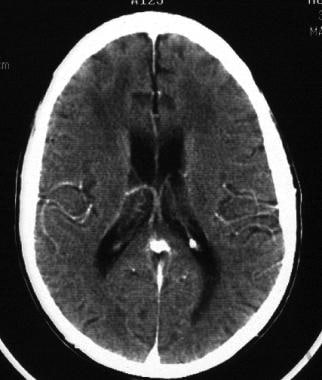 HIV-1 encephalopathy and AIDS dementia complex. CT scan of the brain of a patient with HIV associated neurocognitive disorder (HAND) shows diffuse atrophy and ventricular enlargement and attenuation of periventricular white matter.
HIV-1 encephalopathy and AIDS dementia complex. CT scan of the brain of a patient with HIV associated neurocognitive disorder (HAND) shows diffuse atrophy and ventricular enlargement and attenuation of periventricular white matter.
Primary infection of the CNS may lead to focal white spots in the white matter on MRI. These resemble white matter abnormalities seen in various other settings such as advanced age, diabetes, and hypertension and should be interpreted with caution. Some degree of atrophy occurs during the latent stage, and, with ongoing disease activity, evidence of interstitial fluid accumulation can be seen that is more pronounced around the ventricles, in the form of hyperintense signals on T2-weighted images (see the image below).
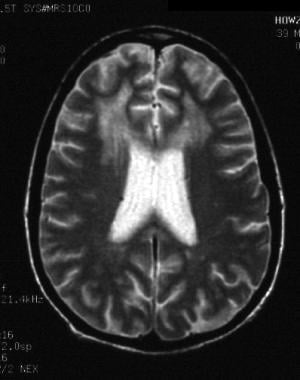 HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). T2-weighted MRI shows ventricular enlargement and large areas of hyperintense signal in the subcortical white matter of both frontal lobes.
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). T2-weighted MRI shows ventricular enlargement and large areas of hyperintense signal in the subcortical white matter of both frontal lobes.
Differential diagnosis includes multiple sclerosis (MS) and small-vessel disease.
Functional MRI
Proton magnetic resonance spectroscopy (MRS) is a functional imaging technique that measures brain metabolites. Neuronal injury is confirmed by finding lower N-acetyl aspartate (NA) levels (a marker of neuronal metabolism) in the frontal white matter. [12]
In the basal ganglia and white matter, where gliosis and inflammatory changes are noted, the level of choline-containing metabolites, which is a marker of glial metabolism, is increased.
Magnetic resonance spectroscopy (MRS) is recognized as a valuable biomarker for assessing brain injury in HIV, providing insights into neurocognitive impairment at the cellular level. [32] MRS findings indicate increased choline (Ch) and myo-inositol (Mi) in the frontal white matter (FWM) and basal ganglia (BG) during early stages of cognitive decline, with later stages showing reduced N-acetylaspartate (NAA), indicating compromised neuronal integrity. Creatine concentrations, which vary with the severity of dementia, are sensitive markers when measured absolutely or relative to internal water. Established initially by Chang et al in 2002, subsequent studies have noted changes in metabolites based on creatine levels, suggesting this should be considered in clinical guidelines.
MRS changes correlating with improvements from combination antiretroviral therapy (cART) can take 6–12 months to manifest. [32] Studies using MRS to measure excitotoxic pathways (via glutamate and glutamine, Glx) and neuroinhibitory pathways (via gamma-aminobutyric acid, GABA) indicate these are among the earliest detectable changes. A meta-analysis by Chelala et al, reviewing 61 spectroscopic studies, found consistent patterns in chronic HIV infection: lower total NAA to creatine ratios (tNAA/tCr), higher total choline to creatine ratios (tCh/tCr), and higher myo-inositol to creatine ratios (mI/tCr). [33] However, the role of creatine in these measurements needs independent assessment, and interpretations remain challenging due to variations in treatment, infection duration, and comorbidities. Overall, MRS is a crucial tool for identifying HAND, monitoring response to cART, and understanding the neuropathogenesis in HIV infection.
Chang et al showed that even in the asymptomatic stage of ADC, MRS demonstrates metabolite changes in the basal ganglia and frontal white matter. In the absence of clinically recognizable symptoms, elevated glial marker, myoinositol-to-creatinine ratio (MI/Cr), is seen in the white matter, indicating early HIV brain disease. [34, 35]
Patients with ADC have elevated MI/Cr and choline-to-creatinine ratio (Cho/Cr) in the basal ganglia and white matter, relative to the asymptomatic group. However, compared with controls, patients with ADC have decreased NA/Cr ratio, which is a neuronal marker. The decreased NA/Cr ratio in HAND is more profound in younger persons. This indicates that in older individuals, the metabolic changes seen may be a combination of age and HIV infection. [34, 35]
Research imaging tools
Position emission tomography (PET) may reveal abnormalities in cortical metabolism. This method is not widely available and is most useful as a research tool. PET scanning may also be useful in very difficult cases to help exclude CNS lymphoma, which shows increased uptake, whereas the lesions of AIDS dementia do not.
Single-photon emission computed tomography (SPECT) may reveal abnormalities in cerebral blood flow. This method is most useful for research rather than as a routine diagnostic tool.
Electroencephalography
Patients with subclinical seizures may present with symptoms that mimic dementia. Consider performing an EEG to help exclude this type of pseudodementia.
EEG findings may be normal in early dementia or may demonstrate diffuse slowing. However, this finding is nonspecific and is present in persons with dementia from any cause (even a metabolic one); therefore, it does not help in making an etiologic diagnosis.
Neuropsychological Testing
Neurocognitive testing plays a vital role in diagnosing HIV-associated neurocognitive disorders (HAND). [23] A detailed neuropsychological battery evaluates multiple cognitive areas such as attention, memory, executive functions, language, and visuospatial skills. Screening tools like the International HIV Dementia Scale (IHDS) and the Montreal Cognitive Assessment (MoCA) are used to pinpoint individuals who might need more comprehensive evaluation. Formal neuropsychological assessments, including the HAND diagnostic algorithm, offer an in-depth analysis of cognitive impairments.
Neuropsychological testing can be used for early screening of asymptomatic, high-risk patients (ie, those with high viral load and low CD4 count [36] ) and for follow-up evaluations. Formal neuropsychological testing results may be normal in minor cognitive motor disorder (MCMD).
Early in the course, impairment of working memory is noted in bedside testing such as digit and word reversals and serial sevens.
In the early stages of the disease, mental status examination findings may be entirely normal. In such cases, neuropsychological testing is especially useful and can help to detect mild, early cognitive abnormalities. This testing can help to quantify and determine the specific pattern of the cognitive abnormality.
Several neuropsychological screening tests have been proposed; the most widely accepted is the modified HIV dementia scale, although this scale has been found to be sensitive only for the most severe forms of impairment. The scale consists of 4 subsets that target memory (ie, recall, registration), psychomotor speed, constructional ability, and concentration. A total of 12 points can be earned, and a score lower than 6 points is considered abnormal. The test takes 10 minutes to administer and can be given by a nonneurologist. These tests are useful diagnostic adjuncts, but the results cannot solely determine the presence of HAND. Because false-negatives can occur with screening tests, a patient with a negative screening test may require more in-depth neuropsychological testing. [37]
In advancing disease, tests that explore the following abilities may be helpful:
-
Motor ability (ie, Finger Tapping Test, Grooved Pegboard Test)
-
Concentration (ie, Continuous Performance Test, Trail Making Test A and B)
-
Processing (ie, Trail Making Test A and B, Choice Reaction Time)
-
Memory/learning (ie, Weschler Memory Scale, California Verbal Learning Test)
-
Abstraction (ie, Wisconsin Card-Sorting Test)
-
Speech/language (ie, Boston Naming Test, Verbal Fluency Test)
Histologic Findings
The hallmark is HIV encephalitis in the white and subcortical gray matter. These changes are noted in 20-90% of patients. Some patients show only minimal changes. Atrophy typically is in a frontotemporal distribution. Diffuse myelin pallor may be present but is more commonly due to changes in the blood-brain barrier than to demyelination. Vacuolation may be observed.
Cortical neuronal loss is noted in 18-50% of patients. Subcortical neuronal loss (substantia nigra) is noted in 25%. Reduced synaptic density and dendritic arborization may be observed. Some neurons and astrocytes appear to die by apoptosis.
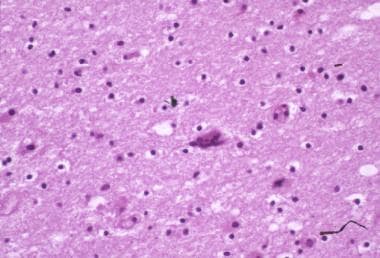
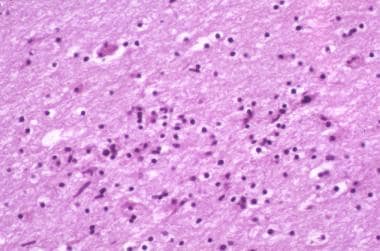
Brain tissue may be infiltrated by microglia, macrophages, lymphocytes, and multinucleated giant cells (see the images below). Activated glial cells are twice as numerous in brains of patients with AIDS as in brains of controls. Microgliosis may be diffuse or form clumps or nodules, often in a perivascular pattern in the white and subcortical gray matter.
Infected cells are associated consistently with macrophages/microglia and endothelial cells. Less commonly observed are astrocytes and neurons with restricted expression of HIV genes.
 HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph shows perivascular and parenchymal infiltrates of lymphocytes and macrophages. These often form microglial nodules. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph shows perivascular and parenchymal infiltrates of lymphocytes and macrophages. These often form microglial nodules. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
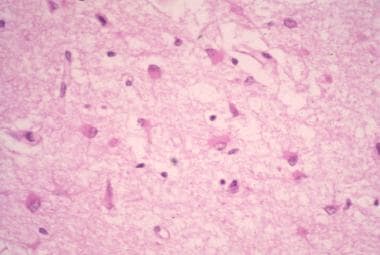 HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph illustrates the intense astrogliosis that is characteristic of HIV encephalitis. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph illustrates the intense astrogliosis that is characteristic of HIV encephalitis. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Antiretroviral and Other Therapies
Addressing the neurological complications of HIV-associated neurocognitive disorders (HAND) necessitates the development of therapeutic strategies including adjunctive neuroprotective approaches, FDA-approved drugs, and optimized combination antiretroviral therapy (cART). [38] These strategies aim to combat factors such as ongoing brain inflammation, low-level HIV-1 replication in microglia, and poor drug penetration across the blood-brain barrier (BBB), which contribute to the persistence of severe conditions like HIV-associated dementia (HAD).
Despite the effectiveness of cART, the challenge remains to enhance drug delivery to the central nervous system (CNS) and to develop new treatments targeting neurotoxic HIV proteins and cellular reservoirs. Promising therapies such as IFNβ and maraviroc are under investigation, and novel delivery systems such as nanodiamond or nanogel formulations are being explored to improve treatment efficacy. Ongoing clinical trials, including those testing agents like baricitinib, are crucial for advancing understanding of how to effectively manage and potentially reduce neurocognitive impairment in HIV-1 infected individuals.
Antiretrovirals
The blood-brain barrier (BBB) is a semipermeable border composed of endothelial cells, along with HIV-susceptible astrocytes and pericytes, that regulates the entry of substances from the bloodstream into the CNS. [9, 23] It blocks pathogens and large or hydrophilic molecules while allowing smaller or hydrophobic molecules to pass. Antiretroviral drugs' (ARVs') penetration into the CNS depends on factors such as molecular weight and protein binding, with stronger protein-bound drugs penetrating more slowly. Transport mechanisms at the BBB, such as p-glycoprotein, also influence drug efflux, affecting treatment efficacy for CNS disorders.
Studies show that ARV penetration into the cerebrospinal fluid (CSF) does not always correlate with brain tissue penetration due to differences in drug dynamics at the BBB. [9] The "free drug hypothesis" suggests that active transport of ARVs can disrupt passive unbound drug movement, leading to varying ARV concentrations in plasma, CSF, and brain tissues. Methodological challenges, such as adsorption of non-polar ARVs to collection tubes, indicate that re-evaluation of ARV levels in CSF might be necessary, especially in virologically suppressed individuals.
Maraviroc, an antiretroviral medication, functions as a CCR5 antagonist, blocking this receptor to prevent the entry of the HIV-1 virus into target cells. [38] Although maraviroc exhibits limited CNS penetration and intra-brain distribution, it has low neurotoxicity and shows some enrichment in gut-associated lymphocytic tissue (GALT). This is particularly relevant in HIV-associated dementia (HAD), where endotoxins can accumulate in the intestine. Thus, maraviroc's properties make it a viable option for treating or preventing HAD by protecting GALT. Previous in vitro studies have demonstrated that maraviroc can inhibit macrophage migration in response to CCL2, contributing to an anti-inflammatory response. However, to fully assess its therapeutic potential and effects on inflammatory markers, new clinical studies with more sensitive assessments and larger sample sizes are necessary.
Highly active antiretroviral therapy (HAART)
Highly active antiretroviral therapy (HAART) is essential for treating HIV-related cognitive disorders, significantly reducing the incidence of dementia from over 50% to 10% and improving neuropsychological outcomes. Early and aggressive treatment with HAART, which suppresses viral replication, is crucial in preventing severe outcomes like HIV dementia [39] and shows sustained improvements in patients. [40]
Despite effective viral control, some patients still progress, possibly due to poor CNS penetration of antiretroviral drugs, as indicated by the Cerebral Penetration Effective Index (CPE). Other contributing factors might include comorbid conditions such as drug use and head trauma, and social determinants of health. Certain antiretrovirals like stavudine and zidovudine have better CNS penetration, but the clinical significance of this remains uncertain. [41] There is no recommendation to adjust HAART based on CNS penetration for cognitive status. [42]
Nanomedicine offers promising strategies to enhance drug delivery to the CNS, potentially overcoming barriers like the BBB. Despite these advancements, the reversal of neurologic deficits often occurs independently of the CNS penetration of specific drugs.
When HAART fails, alternative treatments should be considered, although clinical trials have not found any adjunctive treatments superior to HAART alone. Addressing metabolic causes of cognitive decline and considering nutritional therapies may also benefit patients with cognitive symptoms related to HIV. Future research may explore therapies targeting inflammatory processes with monoclonal antibodies.
Antioxidative Drugs
Dimethyl fumarate (DMF) is an antioxidative medication that influences the kelch-like ECH-associated protein 1 pathway, resulting in the upregulation of antioxidant genes. [23, 38, 43] Additionally, DMF inhibits the nuclear factor B (NF-kB) pathway, which is crucial for initiating HIV-1 transcription. By blocking HIV-1's ability to detect immune cell activation, DMF may help prevent neurodegeneration. In an HIV-positive monocyte-derived macrophage model, DMF has been shown to induce antioxidant responses, inhibit HIV replication, and decrease the release of neurotoxins from infected cells. However, a significant limitation of DMF is its potential to induce apoptosis in specific T-cell subsets. Consequently, there is a risk for progressive multifocal leukoencephalopathy, necessitating close monitoring of patients receiving this treatment in clinical trials.
Other antioxidants include the following:
-
N-Acetylcysteine: This antioxidant shows promise in reducing oxidative stress and inflammation in the brain. It is being studied for its potential to alleviate cognitive impairments in individuals with HIV-associated neurocognitive disorders (HAND).
-
Coenzyme Q10: Known for its neuroprotective properties, Coenzyme Q10 has been researched for its ability to prevent oxidative damage to neurons and potentially slow the progression of HAND.
-
Omega-3 Fatty Acids: Present in fish oil, Omega-3 fatty acids have anti-inflammatory effects and are being investigated for their potential cognitive benefits in managing HAND.
Psychiatric Medications
Paroxetine, a selective serotonin reuptake inhibitor, was found to offer neuroprotection against gp120 and Tat neurotoxicity when used alone or in combination with fluconazole, an oral antifungal medication, according to a 2014 study by Meulendyke et al. [38, 44] The research demonstrated that daily oral administration of fluconazole and paroxetine to simian immunodeficiency virus-infected rhesus macaques provided beneficial neuroprotective effects. However, this combination did not reduce cellular stress, decrease levels of inflammatory markers such as CCL2 and IL-6 in the central nervous system, or inhibit viral replication in the brain, cerebrospinal fluid, or peripheral nervous system.
Both paroxetine and fluconazole are approved for human use, and their combination has been employed as a placebo to mitigate HIV-associated dementia (HAD). Patients treated with paroxetine exhibited improved cognitive functions, enhancing daily activities like verbal fluency, visual attention, and task switching. Conversely, fluconazole did not show any improvement in cellular stress markers or cognitive functions. Given these findings, further research into the role of paroxetine in HAD treatment is warranted, especially since it is well-tolerated in humans and has shown potential in improving certain cognitive functions in patients with HIV-1.
Psychostimulants are a class of medications that can be used to manage cognitive deficits in HIV-associated neurocognitive disorders (HAND), particularly issues with attention and concentration. [23]
Methylphenidate is often prescribed for attention deficit hyperactivity disorder (ADHD); methylphenidate has also been explored for its effectiveness in improving attention and cognitive function in individuals with HAND. Modafinil has been studied for its ability to increase alertness and cognitive performance in those affected by HAND.
Anti-Inflammatory Drugs
Among the various anti-inflammatory medications on the market, minocycline and meloxicam stand out for their potential roles in treating HIV-associated dementia (HAD). [38] Minocycline, an alternative anti-inflammatory treatment, has shown potent effects in inhibiting microglia activation based on in vitro brain inflammation models. It exhibits neuroprotective properties by reducing apoptosis, diminishing the release of pro-inflammatory cytokines, and lowering nitric oxide production. Due to its highly lipophilic nature, minocycline can readily cross the BBB, positioning it as a promising candidate for HAND therapy. Previous studies have demonstrated its anti-inflammatory effects, notably a study where minocycline administered intracerebroventricularly to gp120-expressing rats significantly reduced protein and mRNA levels of inflammatory markers.
On the other hand, meloxicam, a nonsteroidal anti-inflammatory drug (NSAID), works by selectively inhibiting COX-2 enzymes, thereby reducing prostaglandin production and inflammation. It is used in both veterinary and human medicine and is FDA-approved for treating osteoarthritis. Interestingly, meloxicam has been shown to decrease CCL2 expression, which is upregulated by HIV-1 proteins and associated with depression-like behaviors in rats due to changes in neuroinflammation and cell proliferation. However, despite these findings, meloxicam did not alleviate depressive behaviors in these models, leaving its potential role in HAD therapy uncertain.
Interferons
Interferon alpha (IFNα) and interferon beta (IFNβ) are type-I interferon protein cytokines secreted by host immune cells to inhibit viral replication, although their regulation can be inconsistent during the early stages of infection. [38] Research has shown that type-I interferons play a dual role in either reducing or exacerbating neuroinflammation associated with HIV-1 infection. Recent in vitro studies on rat cerebrocortical cells have indicated that IFNβ treatment increases the expression of several chemokines (CCL5, CCL4, CCL3, and CXCL10), with CCL4 playing a key role in mediating IFNβ's neuroprotective effects.
Follow-up
Patients need close follow-up because of progressive dementia, unavoidable polypharmacy with possibly toxic drug levels (in particular, free drug levels), and possible development of seizures and psychosis. Patients may become incapable of self-care and require hospice care.
HIV RNA levels and CD4+ T-cell counts should be evaluated periodically to monitor clinical response and treatment resistance. HIV RNA serum levels generally reflect CSF levels until late in disease when different HIV strains may be present. [45]
Neuropsychological testing should be performed at regular intervals to monitor for improvement and responses to therapy.
Serial MRI may show decreased brain atrophy and improvement in white matter changes when patients respond to HAART.
HAART should be continued indefinitely in essentially all cases.
Depending on the severity and manifestations, patients may require nursing home placement and hospice care. Patients with severe dementia and those who are ematiated are at risk for pressure injuries.
Psychotic features may require psychiatric consultation. Although seizures are rare, their management is difficult, because several antiseizure drugs affect blood levels of antiretroviral drugs.
Patient Competence and Decision Making
Patients should be encouraged to prepare a living will or assign power of attorney early in their disease process. Issues of competence to make medical decisions must be addressed.
Patients may reject proposed treatments. A person’s right to autonomy cannot be challenged, provided that they have the competence to understand the risks and benefits of the treatment offered.
Competence to consent to medical treatment is defined as the “mental capacity necessary to comprehend the risks and benefits of a proposed medical treatment and its alternatives.” The only exception to this situation would be a medical emergency in which the hospital or physician may obtain consent from a surrogate, a close family member, or a spouse. Bioethics consultations may be helpful.
Only a mental health professional psychiatric crisis team, state-designated mental health professional, or peace officer can authorize involuntary hospitalization. Within 72 hours, the patient must be evaluated for mental capacity and grave disability. Grave disability is determined by the ability of the person to provide basic food, clothing, and shelter for himself or herself. If the patient is determined to be gravely disabled, the hold can be extended for 14 days, and the superior court can be petitioned for appointment of a conservator.
HIV-associated dementia may jeopardize the patient’s ability to safely operate a motor vehicle. In such cases, the clinician has a duty to advise both the patient and the Department of Motor Vehicles that it is unsafe for the patient to drive. Some patients may have severe sensory loss related to HIV-associated peripheral neuropathy and thus may be unsafe to drive on that basis. [10]
Consultations
The care of patients with AIDS dementia complex (ADC) is best accomplished with a team approach, drawing upon the expertise of specialists in various fields. A specialist with expertise in HIV (an infectious diseases or general practice physician) should oversee the antiretroviral regimen. Like all patients with HIV disease, patients with HAND require a HAART regimen that is clinically, virologically, and immunologically effective.
Psychiatric and/or psychological consultations are often indicated, as patients with ADC commonly exhibit agitation, anxiety, fatigue, depression, and other psychiatric manifestations. Psychotherapy may be helpful for patients with mild-to-moderate dementia to help them understand, mourn, and adapt to this new impairment in functioning.
As the population of patients with HIV ages, dementia is a risk, because it occurs late in life; older patients with HIV may develop Parkinson disease, frontotemporal dementia, Lewy body dementia, or Alzheimer disease unrelated to HIV. A neurologic workup by a neurologist specializing in neurobehavioral disorders may help to sort out the etiology of cognitive impairment.
The physical therapist and occupational therapist each plays a vital role in trying to maximize the functional capacity of the patient.
-
HIV-1 encephalopathy and AIDS dementia complex. CT scan of the brain of a patient with HIV associated neurocognitive disorder (HAND) shows diffuse atrophy and ventricular enlargement and attenuation of periventricular white matter.
-
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). T2-weighted MRI shows ventricular enlargement and large areas of hyperintense signal in the subcortical white matter of both frontal lobes.
-
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph shows perivascular and parenchymal infiltrates of lymphocytes and macrophages. These often form microglial nodules. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
-
HIV-1 encephalopathy and HIV associated neurocognitive disorder (HAND). Photomicrograph illustrates the intense astrogliosis that is characteristic of HIV encephalitis. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
-
HIV-1 encephalopathy and AIDS dementia complex. Multinucleated giant cells, as shown here, are a hallmark of HIV encephalitis and harbor the virus. Image contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Tables
What would you like to print?
- Overview
- Pathophysiology
- Epidemiology
- Prognosis
- Patient Education
- Presentation
- Physical Examination
- Diagnostic Criteria
- Staging of AIDS Dementia Complex
- Neurologic Examination in Pediatric Patients
- Differential Diagnosis
- Overview of Workup
- Cerebrospinal Fluid Analysis
- Neuroimaging
- Electroencephalography
- Neuropsychological Testing
- Histologic Findings
- Antiretroviral and Other Therapies
- Follow-up
- Patient Competence and Decision Making
- Consultations
- Show All
- Media Gallery
- References