Background
Viral infections in pregnancy are major causes of maternal and fetal morbidity and mortality. Infections can develop in the neonate transplacentally, perinatally (from vaginal secretions or blood), or postnatally (from breast milk or other sources). The clinical manifestations of neonatal infections vary depending on the viral agent and the gestational age at exposure. The risk for infection is usually inversely related to gestational age at acquisition, some resulting in a congenital malformation syndrome.
Infections known to produce congenital defects have been described with the acronym TORCH (Toxoplasma, others, rubella, cytomegalovirus [CMV], herpes). The "others" category has rapidly expanded to include several viruses known to cause neonatal disease.
Traditionally, the only viral infections of concern during pregnancy were those caused by rubella virus, CMV, and herpes simplex virus (HSV). Other viruses now known to cause congenital infections include parvovirus B19 (B19V), varicella-zoster virus (VZV), West Nile virus, measles virus, enteroviruses, adenovirus, human immunodeficiency virus (HIV), and Zika virus.
Also of importance is hepatitis E virus because of the high mortality rate associated with infection in pregnant women. Additionally, lymphocytic choriomeningitis virus (LCMV) has been implicated as a teratogenic rodent-borne arenavirus.
Worldwide, congenital HIV infection is a major cause of infant and childhood morbidity and mortality, responsible for an estimated 4 million deaths since the start of the HIV pandemic. The breadth and depth of this problem is beyond the scope of this article.
With emerging concerns for an influenza pandemic, attention has also been directed to the effects of influenza on pregnant women. Pregnant women are more likely to develop severe disease, perhaps related to physiologic changes in pregnancy, such as decreased lung capacity, increased oxygen needs, and increased heart rate. Currently, inactivated influenza vaccine is recommended in all trimesters of pregnancy. [1] One study found that influenza vaccination of high-risk pregnant patients also provides some protective immunity for newborns and reduces subsequent hospitalizations in the infants. [2] Influenza has historically been shown to produce significant morbidity and mortality in this population.
During pregnancy, patients are at elevated risk for severe disease associated with COVID-19. They are more likely to be admitted to an intensive care unit and require invasive ventilation than nonpregnant patients. [3]
There is some suggestion that pregnant people may be more susceptible to severe disease and death from Ebola.
Zika virus
Zika virus, first introduced in South America in 2015, has since spread throughout the Americas. The virus has been shown to cause severe congenital abnormalities, including microcephaly. There is an ongoing Zika virus outbreak in the Americas, the Caribbean, and the Pacific.
Cytomegalovirus
Cytomegalovirus (CMV) infection is the most common perinatal viral infection in the United States. CMV is a double-stranded DNA herpes virus and represents the most common congenital viral infection. The CMV seropositivity rate increases with age. Geographic location, socioeconomic class, and work exposure are other factors that influence the risk of infection. CMV infection requires intimate contact through saliva, urine, and/or other body fluids. Possible routes of transmission include sexual contact, organ transplantation, transplacental transmission, transmission via breast milk, and blood transfusion (rare).
Primary, reactivation, or recurrent CMV infection can occur in pregnancy and can lead to congenital CMV infection. Transplacental infection can result in intrauterine growth restriction, sensorineural hearing loss, intracranial calcifications, microcephaly, hydrocephalus, hepatosplenomegaly, delayed psychomotor development, and/or optic atrophy.
Vertical transmission of CMV can occur at any stage of pregnancy; however, severe sequelae are more common with infection in the first trimester, while the overall risk of infection is greatest in the third trimester. The risk for transmission to the fetus in primary infection is 30-40%. Most (90%) CMV infections cause no symptoms, but 10% result in signs and symptoms such as microcephaly, thrombocytopenia, hepatosplenomegaly, intrauterine growth restriction, or a combination thereof.
Thirty percent of infants with severe CMV infection die; among survivors, more than half eventually develop neurologic sequelae, including microcephaly, intellectual disability, and/or sensorineural hearing loss. Seven percent of asymptomatic neonates develop sensorineural hearing loss or developmental delays during the first 2 years of life. [4] Five percent eventually develop microcephaly and neuromuscular defects, and 2% develop chorioretinitis. Congenital hearing loss is the most common sequela of recurrent CMV infection.
Herpes simplex virus
Thirty to sixty percent of individuals receiving obstetric care have serologic evidence of past HSV infection. Although both HSV-1 and HSV-2 may cause neonatal herpes, HSV-2 is responsible for 70% of cases.
Neonatal herpetic infection is defined as infection within 28 days of birth. Ninety percent of infections are perinatally transmitted in the birth canal. HSV infection acquired in this manner carries a 70% risk for dissemination and is associated with three distinct syndromes, each with its own typical outcome. The first and most common (45%) is localized skin, eye, or mouth disease. Approximately 30% of cases manifest as central nervous system (CNS) disease, including meningitis or encephalitis, with evidence of HSV DNA in the cerebrospinal fluid (CSF). Finally, 25% of neonatal herpetic infections manifest as disseminated disease that involves multiple organs. Initial symptoms of this disease usually present during the first 4 weeks of life.
Approximately 10% of infections are congenital, usually a consequence of the mother acquiring primary HSV infection during pregnancy and the fetus acquiring the infection transplacentally or via an ascending infection from the cervix. Intrauterine infection is associated with intrauterine growth restriction, preterm labor, and miscarriage. [5, 6] The risk for neonatal herpes and death is highest in infants born to mothers who have not seroconverted by the time of delivery.
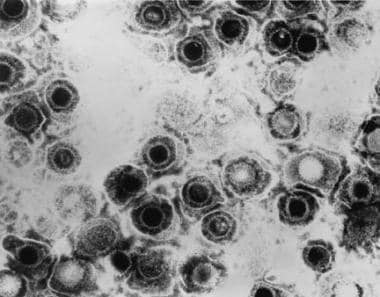 Viral infections and pregnancy. Transmission electron micrograph of herpes simplex virus. Some nucleocapsids are empty, as shown by penetration of electron-dense stain. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 281.
Viral infections and pregnancy. Transmission electron micrograph of herpes simplex virus. Some nucleocapsids are empty, as shown by penetration of electron-dense stain. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 281.
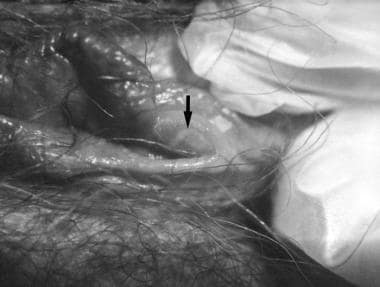 Viral infections and pregnancy. Blisters on the vulva due to a recurring herpes II (HSV-2) virus infection. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 2319.
Viral infections and pregnancy. Blisters on the vulva due to a recurring herpes II (HSV-2) virus infection. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 2319.
Rubella
Rubella is one of the more teratogenic viruses. Congenital rubella syndrome (CRS) is characterized by intrauterine growth restriction, intracranial calcifications, microcephaly, cataracts, cardiac defects (most commonly patent ductus arteriosus or pulmonary arterial hypoplasia), neurologic disease (with a broad range of presentations, from behavior disorders to meningoencephalitis), osteitis, and hepatosplenomegaly.
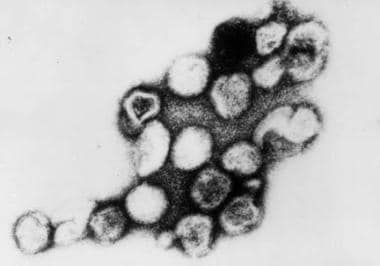 Viral infections and pregnancy. Transmission electron micrograph of rubella virus. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 269.
Viral infections and pregnancy. Transmission electron micrograph of rubella virus. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 269.
Neonates with rubella may have a "blueberry muffin" appearance caused by purpuric skin lesions that result from extramedullary hematopoiesis. Heart defects in these infants include ventricular septal defects, patent ductus arteriosus, pulmonary stenosis, and coarctation of the aorta. The presentation of rubella at birth varies greatly. Most of these complications develop in infants born to mothers who acquire rubella infection during the first 16 weeks of pregnancy. Ninety percent of infants present with some finding of congenital rubella if infection occurs within the first 12 weeks, and 20% present with congenital disease if the infection occurs between weeks 12 and 16. [7] Cataracts results when infection occurs between the third and eighth week of gestation, deafness between the 3rd and 18th week, and heart abnormalities between the 3rd and 10th week. [8]
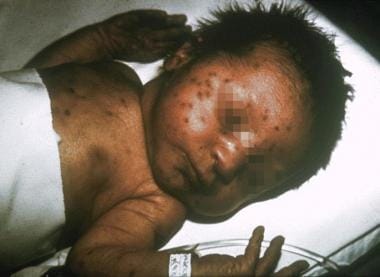 Viral infections and pregnancy. Infant with congenital rubella and blueberry muffin skin lesions. Lesions are sites of extramedullary hematopoiesis and can be associated with several different congenital viral infections and hematologic diseases. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 713.
Viral infections and pregnancy. Infant with congenital rubella and blueberry muffin skin lesions. Lesions are sites of extramedullary hematopoiesis and can be associated with several different congenital viral infections and hematologic diseases. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 713.
Parvovirus B19
B19V causes erythema infectiosum (fifth disease). Although most adults with B19V infection are asymptomatic, the effects of this virus on the fetus are much greater and include miscarriage, fetal anemia, hydrops fetalis, myocarditis, and/or intrauterine fetal death. Infection occurs most frequently in the winter and spring. B19V infection accounts for 15% to 20% of cases of nonimmune hydrops fetalis. Thirty to forty percent of pregnant women are seronegative for B19V and are thus susceptible to infection.
Various studies have estimated that 3-14% of intrauterine fetal deaths occur in the setting of B19V infection. Second-trimester infections have been studied most frequently because infection in this trimester carries a 1-3% risk for hydrops; however, infection in any trimester may result in intrauterine fetal loss. The critical period for the development of fetal hydrops is when maternal B19V infection is acquired between the 13th and 16th week of gestation, possibly because of the relative immaturity of the fetal immune response, as well as the shortened life span of the red blood cells at this gestational age.
Varicella-zoster virus
VZV is a common virus that carries risks for both the mother and fetus during pregnancy. Morbidity and mortality rates associated with VZV infection are much higher in adults than in children. Primary varicella infection during pregnancy is considered a medical emergency. Pneumonitis due to VZV infection is 25 times more common in adults than in children; in the third trimester, the risk for life-threatening ventilatory compromise is significant, with a mortality rate of 14%. Before the development of antiretrovirals, pneumonitis in pregnant women carried a mortality rate of 45%. Other risk factors for the development of pneumonitis include smoking and a large lesion burden (>100 lesions). [9]
Congenital varicella syndrome (CVS) results in spontaneous abortion, chorioretinitis, cataracts, limb atrophy, cerebral cortical atrophy, and/or neurologic disability. Spontaneous abortion has been reported in 3-8% of first-trimester VZV infections, and CVS has been reported in 12%. [10] Acquisition of infection by the mother in the perinatal period, specifically 5 days prior to delivery or 2 days afterward, poses a risk for severe neonatal varicella, which carries a mortality rate of 30%. Infection at this time prevents development of maternal antibodies that avert transplacental transfer of immunoglobulin G (IgG) antibodies, which confer passive immunity to the fetus.
Enterovirus
Enterovirus infections are not believed to cross the placenta and cause fetal disease. [11] However, some studies have linked coxsackievirus and echovirus to miscarriage, neurodevelopmental delay, myocarditis, and cortical necrosis. [12, 13] One study linked the presence of coxsackievirus in the third trimester with respiratory failure and global cognitive defects. [14]
Measles virus
Measles virus infection (rubeola) during pregnancy, as with VZV infection, tends to be severe, with pneumonitis predominating. Although it is not known to be teratogenic, rubeola has been associated with spontaneous abortion, premature labor, and low birth weight. Neonates born to mothers with active measles virus infection are at risk of developing neonatal measles, but no congenital syndrome has been described. [15]
Lymphocytic choriomeningitis virus
LCMV has been associated with sporadic cases of congenital infection worldwide. Affected infants demonstrate chorioretinitis, hydrocephalus, intellectual disability, and/or visual impairment; in addition, intrauterine death is possible. Unlike congenital CMV and rubella infections, hearing deficits and hepatosplenomegaly are rarely seen in congenital LCMV.
Other viruses
Other viruses postulated to cause congenital infections include echovirus, hepatitis B virus, hepatitis C virus, and adenovirus. [16] In 2002, a case of West Nile virus infection in a mother with associated chorioretinitis in her newborn was reported. [17] A causal link has not been determined. Since then, the Centers for Disease Control and Prevention (CDC) has maintained a registry of West Nile virus infections during pregnancy. Other congenital malformations have been described in this registry, but a direct cause-effect relationship has not yet been established. Infants born to mothers who develop symptomatic West Nile virus infection within 3 weeks prior to delivery may develop symptomatic West Nile virus disease shortly after birth.
Influenza poses a significant threat to the health of the mother and infant. Historic reports of the 1918 Spanish flu pandemic and the 1957 Asian flu pandemic reported a mortality rate of approximately 50% among infected pregnant people. [18] During the 2009 H1N1 pandemic, pregnant people accounted for 5% of all deaths and were more likely to be hospitalized than the general population. [19]
Pregnant patients are at increased risk for severe COVID-19 complications, potentially leading to adverse pregnancy outcomes. Although vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is extremely rare, maternal infection has been linked to preterm birth, stillbirth, and miscarriage. [3] A Swedish study found higher rates of respiratory disorders, hyperbilirubinemia, and admission for neonatal care in infants born to mothers who tested positive for SARS-CoV-2. [20]
Pathophysiology
Human CMV is the largest of the beta herpes viruses and can cause lytic and productive infection. Like other herpes viruses, it can be latent and reactivate. CMV infection in pregnancy can be primary (initial acquisition in pregnancy) or recurrent. Vertical transmission can occur transplacentally; in addition, the virus can be transmitted via cervicovaginal secretions at the time of delivery or by ingestion of breast milk postpartum. Transplacental transmission is associated with congenital CMV infection. Maternal shedding at time of delivery is associated with a 50% risk for infection. [21] CMV infection acquired through exposure to infected cervical secretions or human milk is usually asymptomatic and is not associated with neonatal sequelae.
Herpes may be transmitted to the fetus in the peripartum period (as the neonate passes through the birth canal [85%]), via intrauterine transmission (either from ascending infection through the cervical canal or transplacentally [5%]), or via postnatal transmission (10%). Both HSV and VZV have tropisms for neural tissue. Peripartum transmission leads to disseminated disease in 70% of infants and is characterized by skin lesions, encephalitis, and neurological disability. The risk for neonatal herpetic infection is much higher in women with primary infection. Primary infection carries a transmission rate of 25% to 50%, whereas recurrent maternal herpes infection carries a transmission rate of less than 1%. [22] The difference in transmission rates may be due to the presence of antibodies and lower viral loads with recurrent infection.
In addition to miscarriage, B19V can cause fetal anemia due to effects on fetal red blood cell precursors, which can lead to hydrops. B19V has a tropism for the fetal bone marrow and liver, causing apoptosis of erythroid precursors and thus inhibiting erythropoiesis. Fetal liver erythroblasts exhibit viral DNA and pathognomonic changes of B19V infection. The myocardium has also been affected, causing myocarditis and resultant heart failure.
VZV is a DNA herpes virus. Following primary VZV infection, it can remain latent in the dorsal root ganglia. Primary varicella usually confers lifelong immunity. VZV is most often transmitted to the fetus transplacentally; however, ascending infection from lesions in the birth canal has been reported. [23] The mechanism of in utero VZV infection is unknown. Infection of developing nerve bundles may explain limb atrophy and chorioretinitis in CVS.
Rubella is an RNA virus found to infect only humans. It is spread by airborne respiratory secretions and is most common in late winter and early spring. The virus travels from the upper respiratory tract to the cervical lymph nodes and is then disseminated throughout the body. The incubation period is 2 to 3 weeks. Antibodies against rubella do not appear in the serum until after the rash has developed. Fetal infection results from transplacental vertical transmission.
Ebola is a zoonotic infection, and the natural reservoir is thought to be fruit bats. This virus is transmitted person-to-person via direct skin contact or mucous membranes with blood or bloody fluids of infected patients. The incubation period can range from 2 to 21 days.
Zika virus is an arthropod-borne flavivirus that is similar to other flaviviruses such as dengue fever, yellow fever, and West Nile virus. The virus spreads to humans through infected Aedes species of mosquitoes, sexual contact, and perinatal transmission to fetus in pregnancy. Both male-to-female and female-to-male sexual transmissions have been reported. It remains unclear whether pregnant women are more susceptible to infection.
Epidemiology
CMV is the most common virus known to be transmitted in utero, affecting approximately 0.5% to 1.5% of births. [24] Approximately 40% of maternal CMV infections during pregnancy result in congenital infection. [25]
Depending on the demographic population, neonatal herpes infection affects 1 per 1700 to 1 per 12,500 live births. [22] The rate of HSV-2 seroconversion during pregnancy is estimated to be 0.2% to 4%.
The estimated incidence of primary B19V infection in pregnancy ranges from 1% to 5%.
Varicella occurs in approximately one to seven per 10,000 pregnancies. [10]
In 1999, the incidence of rubella was 0.1 per 100,000. The incidence of congenital rubella syndrome has decreased dramatically in the United States because of rubella vaccination; currently, fewer than 50 cases occur each year. [26]
LCMV infection occurs in the Americas and Europe in areas where people are exposed to the host species of hamsters, Mus domesticus and Mus musculus. Infections tend to occur in focal geographic areas in autumn.
Prognosis
Prognosis depends on the viral syndrome and the severity of the initial infection.
Morbidity/mortality
The risk for primary maternal CMV infection leading to congenital CMV infection is approximately 40%. Of neonates with congenital CMV infection, 85% to 90% are asymptomatic at birth, yet 10% to 15% eventually present with developmental, visual, hearing, or dental abnormalities in the first years of life. Of those who are symptomatic at birth, about half will present with some isolated findings, whereas the other half will present with cytomegalic inclusion disease. CMV disease in this group carries a mortality rate of around 30%; up to 80% of affected infants develop late complications, including developmental, visual, or hearing delay.
Morbidity and mortality rates are higher in patients infected with HSV-2 than in those with HSV-1. Neonatal disseminated HSV infection acquired perinatally carries a 65% mortality rate if untreated and a 25% mortality rate if treated.
Congenital varicella syndrome (CVS) carries a 30% mortality rate. [10] Acquisition of varicella infection by the mother in the immediate perinatal period, specifically from 5 days before or 2 days after delivery, poses the greatest risk for severe neonatal varicella infection, as maternal antibodies have not yet developed to confer passive immunity to the fetus. Reactivation of the virus results in zoster infection, commonly known as shingles. No evidence has shown that herpes zoster infection causes a more severe infection in pregnancy or results in congenital malformations.
Fifty to eighty percent of infants exposed to rubella virus within 12 weeks of conception show signs of congenital infection. [26] The rate of congenital infection drops dramatically with advancing gestational age, such that the risk of congenital infection is very small if infection occurs after 18 weeks of gestation.
LCMV infection is rarely fatal in the adult host, but fetal acquisition may lead to intrauterine death.
Up to 20% of pregnant women who acquire hepatitis E develop fulminant hepatic failure.
Historic data from previous pandemics suggest a mortality rate of up to 50% among pregnant women. During the 2009 H1N1 pandemic, pregnant individuals were more likely to be hospitalized and accounted for 5% of all influenza mortality. [27]
Congenital Zika virus infection can be associated with neurologic abnormalities, positional abnormalities, hearing loss, and multiple ocular abnormalities, including, but not limited to, retinal dysplasia, glaucoma, optic nerve abnormalities, and nystagmus. These cannot be detected antenatally.
-
Viral infections and pregnancy. Transmission electron micrograph of herpes simplex virus. Some nucleocapsids are empty, as shown by penetration of electron-dense stain. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 281.
-
Viral infections and pregnancy. Transmission electron micrograph of rubella virus. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 269.
-
Viral infections and pregnancy. Blisters on the vulva due to a recurring herpes II (HSV-2) virus infection. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 2319.
-
Viral infections and pregnancy. Infant with congenital rubella and blueberry muffin skin lesions. Lesions are sites of extramedullary hematopoiesis and can be associated with several different congenital viral infections and hematologic diseases. Image and caption from US Centers for Disease Control and Prevention Public Health Image Library, available at: https://phil.cdc.gov/Phil/search.asp. Use Image ID 713.

