Background
Pleural effusions are a commonly noted in patients diagnosed with pneumonia. They can be noted in 14-44% of patients admitted for pneumonia, and 40% of these cases could be complicated by parapneumonic effusion or abscess. [1] In most patients, treatment with antibiotics leads to resolution; however, some patients may develop a more fibrinous reaction, with the presence of frank pus in the most severe cases (referred to as empyema or empyema thoracis).
Virtually any type of pneumonia (eg, bacterial, viral, or atypical) can be associated with a parapneumonic pleural effusion. However, the relative incidence of parapneumonic pleural effusions varies with the causative organism. Viral pneumonia and mycoplasmal pneumonia cause small pleural effusions in 20% of patients. For thoracic empyema, bacterial pneumonia is the cause in 70%. [2]
Increasingly, empyema thoracis is a complication of previous surgery, which accounts for 30% of cases. Trauma may also be complicated by infection of the pleural space. In the absence of trauma or surgery, the infecting organism may spread from blood or other organs into the pleural space. These infections can develop into subdiaphragmatic abscesses, a ruptured esophagus, mediastinitis, osteomyelitis, pericarditis, cholangitis, and diverticulitis, among other conditions.
Classification
Parapneumonic pleural effusions are classified into three broad groups on the basis of fluid characteristics, which, in turn, provides a reflection on both the severity and natural history of the pleural effusion.
Pathophysiology
The evolution of a parapneumonic pleural effusion (see the image below) can be divided into the following three stages [2] :
-
Exudative
-
Fibrinopurulent
-
Organization
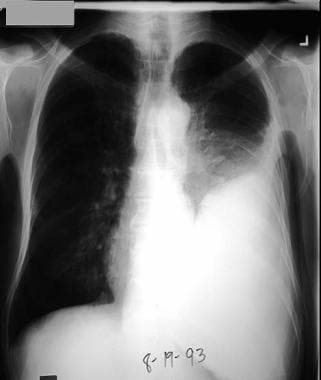 Left pleural effusion developed 4 days after antibiotic treatment for pneumococcal pneumonia. Patient developed fever, left-side chest pain, and increasing dyspnea. During thoracentesis, purulent pleural fluid was removed, and Gram stain showed gram-positive diplococci. Culture confirmed this to be Streptococcus pneumoniae.
Left pleural effusion developed 4 days after antibiotic treatment for pneumococcal pneumonia. Patient developed fever, left-side chest pain, and increasing dyspnea. During thoracentesis, purulent pleural fluid was removed, and Gram stain showed gram-positive diplococci. Culture confirmed this to be Streptococcus pneumoniae.
During the first (ie, exudative) stage, sterile pleural fluid rapidly accumulates in the pleural space. This fluid originates in the interstitial spaces of the lung and in the capillaries of the visceral pleura as a consequence of increased permeability. The pleural fluid has a low white blood cell (WBC) count and a relatively low LDH level. Its glucose and pH levels are within the reference range. These effusions resolve with antibiotic therapy, and chest tube insertion is not required. This stage takes approximately 2-5 days from the onset of pneumonia.
In the second (ie, fibrinopurulent) stage, bacterial invasion of the pleural space occurs, with accumulation of polymorphonuclear leukocytes, bacteria, and cellular debris. A tendency toward loculation and septation exists, pleural fluid pH (< 7.20) and glucose levels are lower (< 60 mg/dL), and LDH levels increase. At this stage, bacteriologic stains or cultures of the pleural fluid can be positive for microorganisms. This stage takes approximately 5-10 days after pneumonia onset.
In the third (ie, organization) stage, fibroblasts grow into the exudates from both visceral and parietal pleural surfaces, producing an inelastic membrane called a pleural peel. the pleural fluid is thick. In an untreated patient, pleural fluid may drain spontaneously through the chest wall (ie, empyema necessitatis [necessitans]). Empyema thoracis may arise without an associated pneumonic process (eg, from esophageal perforation, trauma, a surgical procedure in the pleural space, or bloodstream infection [BSI]). This final stage may take 2-3 weeks to develop.
Etiology
Pneumonia is the leading cause of parapneumonic effusions and empyema thoracis. Increasingly, empyema is also a complication of previous cardiothoracic surgery, which accounts for 30% of cases. The usual organisms are Staphylococcus species and gram-negative bacteria. Trauma can also lead to inoculation and superinfection of the pleural space.
In the absence of trauma or surgery, the infecting organism may have spread from blood or other organs into the pleural space. Possible causes include extension of infections from adjacent or distant sites (eg, ruptured esophagus, mediastinitis, osteomyelitis, pericarditis, cholangitis, diverticulitis, or pericarditis) and subdiaphragmatic abscesses.
Microbiology
The microbiologic etiology of culture-positive parapneumonic pleural effusions has changed over time and varies according to the parapneumonic or nonparapneumonic nature of infection. [3, 4, 5] Before the antibiotic era, Streptococcus pneumoniae was the most common isolate. At present, S pneumoniae and Staphylococcus aureus account for approximately 70% of aerobic gram-positive cultures. Aerobic organisms are isolated slightly more frequently than anaerobic organisms. Streptococcus milleri has also become more common.
Klebsiella, Pseudomonas, and Haemophilus species are the most frequently isolated aerobic gram-negative organisms in this setting; Bacteroides and Peptostreptococcus species are the most commonly isolated anaerobic organisms. Currently, empyema thoracis is most often associated with aspiration pneumonia characterized by a mixed bacterial flora containing both aerobes and anaerobes. [6] S aureus is the organism usually isolated in hospital-acquired empyema and in cases occurring as complications after surgery.
Tuberculous effusions are relatively uncommon in developed nations but are frequently noted worldwide and should be considered in specific populations (eg, patients from regions where tuberculosis [TB] is endemic and patients with a history of TB at risk for reactivation). [7]
Fungal infections are relatively rare as well but should be considered in immunocompromised patients.
Risk factors
Risk factors for empyema thoracis include age (children and elderly persons), debilitation, pneumonia requiring hospitalization, and comorbid diseases, such as bronchiectasis, rheumatoid arthritis, alcoholism, diabetes, and gastroesophageal reflux disease. [2]
A large prospective observational study in the United Kingdom used multivariate regression analysis to identify seven clinical factors predicting the development of complicated parapneumonic pleural effusions or empyema thoracis. [8] An albumin value lower than 30 g/L, a serum sodium value lower than 130 mmol/L, a platelet count higher than 400 × 109/L, a C-reactive protein (CRP) level higher than 100 mg/L, and a history of alcohol abuse or intravenous (IV) drug use were found to be independently associated with the development of complicated parapneumonic pleural effusions or empyema thoracis, whereas a history of chronic obstructive pulmonary disease (COPD) was associated with a decreased risk.
Epidemiology
US and international statistics
Hospital discharge data indicate that in the United States, approximately 1.3 million patients are hospitalized with pneumonia every year. The prevalence of parapneumonic effusions is dependent, in part, on the organism involved.
Overall, pleural effusions are seen in about 35-40% of patients with bacterial pneumonia or anaerobic pneumonia, with a prevalence approaching 60% in pneumococcal pneumonia. Complicated pleural effusions are more common with anaerobic pleuropulmonary infections. This results in an estimated 500,000-750,000 patients with parapneumonic effusions annually. No good estimates are available regarding how many of these patients proceed to complicated effusions or empyema, but in small series, approximately 5-10% have been found require drainage or a surgical procedure.
A study of US hospitalization data found that in 1996, the national hospitalization rate for parapneumonic empyema–related diagnoses was 3.04 per 100,000; by 2008, it had increased to 5.98 per 100,000, and by 2016, it had increased to 11.1 per 100,000. [9] The empyema hospitalizations had a high cost per case ($38,591) and required a prolonged stay (13.8 d). Over the study period, a downward trend in length of stay and a small reduction in associated mortality were not, possibly due to increasing use of innovative treatments (eg, surgery or intrapleural enzymatic therapy).
No good estimates are available on the international incidence of pneumonia. The World Health Organization (WHO) cited a figure of 4.2 million for cases involving death from lower respiratory tract infections in 2004. From this figure, it is possible to extrapolate the incidence of pleural effusions and empyema using a US estimate, but caution is advised because lack of treatment and delayed treatment in developing countries may skew the international incidence upward.
Age-, sex-, and race-related demographics
In a 2021 report from the American College of Chest Physicians (ACCP), 60% of the hospitalizations related to pleural infections were found to occur in the 18- to 64-year-old age group. [9] In the United Kingdom and Europe, this age group accounted for 40% of adult pleural infections. [10] Advancing age leads to an increase in associated comorbidities, including a higher risk of pneumonia and, subsequently, pleural effusions and empyema.
In the 2021 ACCP report, a male predominance in adult pleural infections was noted. [9] Plausible hypotheses for this difference include the following [10, 9] :
-
Gender differences in health-seeking behaviors, with a delayed presentation in males
-
Worse dental hygiene trends
-
Greater frequency of preexisting comorbidities in males
No specific ethnic or racial predisposition is recognized for empyema; however, a larger number of ethnic minorities have limited financial resources, less access to healthcare, and more comorbidities, which, in turn, may increase their risk of pneumonia, pleural effusions, and empyema.
Prognosis
The prognosis for patients with parapneumonic effusion can be defined in terms of four categories derived from the ABC classification published by the ACCP in 2000. [11] This classification is based on three characteristics of pleural fluid—namely, pleural space anatomy (A), fluid bacteriology (B) and fluid chemistry (C)—as follows:
-
Category 1 (very low risk, with no drainage indicated) - A0 (minimal free-flowing effusion < 10 mm on lateral decubitus), Bx (culture and Gram stain results unknown), and Cx (pH unknown)
-
Category 2 (low risk, with no drainage indicated) - A1 (small to moderate-sized free-flowing effusion >10 mm and < one half hemithorax), B0 (negative culture and Gram stain), and C0 (pH ≥7.20)
-
Category 3 (moderate risk, with drainage indicated) - A2 (large free-flowing effusion ≥ one half hemithorax, loculated effusion, or effusion with thickened parietal pleura), B1 (positive culture and Gram stain), and C1 (pH < 7.20)
-
Category 4 (high risk, with drainage indicated) - Presence of frank pus in pleural space
Additional scoring systems, such as RAPID (renal, age, purulence, infection source, and dietary factors), can help identify patients at risk of poor outcome at presentation). [12, 10] This clinical risk score, derived from the first Multicenter Intrapleural Sepsis Trial (MIST1), stratified patients into low-risk (0-2), medium-risk (3-4), and high-risk (5-7) groups. A RAPID score of 3-4 was associated with an odds ratio (OR) of 24.4 for death at 3 months, whereas a score higher than 4 was associated with an OR of 192.4.
Most patients recover in the early stage, but mortality remains approximately 10%. Appropriate antibiotic therapy and early drainage of pleural fluid, when indicated, are crucial for recovery. Approximately 15-25% of patients require surgical intervention, including decortication or an open drainage procedure, especially in categories 3 and 4
Historically, mortality figures for empyema have been reported to range from 11% to 50%. The width of this range is due in part to limited data, with mortality being higher (~50%) at a time when current diagnostic imaging, antibiotics, and drainage options were not readily available. Other complicating factors include cardiac and respiratory comorbidities, immunosuppressive states related to medications or HIV infection, and age. Death rates related to pneumonia are higher in elderly persons and in those with the outlined underlying comorbidities. Reports from the 2000s have estimated mortality from pneumonia and complicated pleural effusions to be in the range of 7-10%. [13]
-
Left pleural effusion developed 4 days after antibiotic treatment for pneumococcal pneumonia. Patient developed fever, left-side chest pain, and increasing dyspnea. During thoracentesis, purulent pleural fluid was removed, and Gram stain showed gram-positive diplococci. Culture confirmed this to be Streptococcus pneumoniae.
-
Left lateral chest radiograph shows large left pleural effusion.
-
Right lateral decubitus chest radiograph shows free-flowing pleural effusion, which should be sampled with thoracentesis for pH determination, Gram stain, and culture.
-
CT scan of thorax shows loculated pleural effusion on left and contrast enhancement of visceral pleura, indicating etiology is likely empyema.
-
Chest CT scan with intravenous contrast in patient with mixed Streptococcus milleri and anaerobic empyema following aspiration pneumonia, showing thickened contrast-enhanced pleural rind, high-density pleural effusion, loculation, and septation. Thoracentesis yielded foul-smelling pus.
-
Chest CT scan with intravenous contrast in patient with mixed Streptococcus milleri and anaerobic empyema following aspiration pneumonia, 3 days following thoracostomy and intrapleural fibrinolysis (Reteplase).
-
Chest CT scan with intravenous contrast (axial, coronal, and sagittal views) of alcoholic male patient with anaerobic empyema demonstrating split pleura sign.
-
Ultrasonogram of pleural space shows densely septated loculation in pleural space.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Guidelines
- Medication
- Antibiotics, Lincosamide
- Carbapenems
- Cephalosporins, 2nd Generation
- Cephalosporins, 3rd Generation
- Cephalosporins, 4th Generation
- Enzymes, Mucolytic
- Fluoroquinolones
- Glycopeptides
- H pylori Agents
- Macrolides
- Nitroimidazoles
- Oxazolidinones
- Penicillins, Amino
- Penicillins, Extended-Spectrum
- Penicillins, Natural
- Thrombolytics
- Show All
- Media Gallery
- References


