Practice Essentials
Pelvic inflammatory disease (PID) is an infectious and inflammatory disorder of the upper female genital tract, including the uterus, fallopian tubes, and adjacent pelvic structures. Infection and inflammation may spread to the abdomen, including perihepatic structures (Fitz-Hugh−Curtis syndrome). The classic high-risk patient is a menstruating woman younger than 25 years who has multiple sex partners, does not use contraception, and lives in an area with a high prevalence of sexually transmitted disease (STD).
Signs and symptoms of pelvic inflammatory disease
The diagnosis of acute PID is primarily based on historical and clinical findings. Clinical manifestations of PID vary widely. Many patients exhibit few or no symptoms, whereas others have acute, serious illness. The most common presenting complaint is lower abdominal pain. Many women report an abnormal vaginal discharge. (See Presentation.)
Diagnosis of pelvic inflammatory disease
The differential diagnosis includes appendicitis, cervicitis, urinary tract infection, endometriosis, ovarian torsion, and adnexal tumors. Ectopic pregnancy can be mistaken for PID; indeed, PID is the most common incorrect diagnosis in cases of ectopic pregnancy. Consequently, a pregnancy test is mandatory in the workup of women of childbearing age who have lower abdominal pain. (See DDx.)
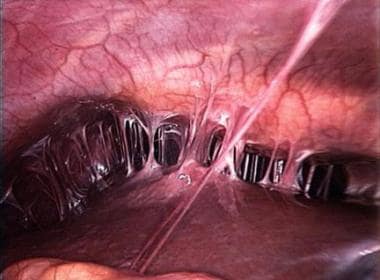
PID may produce tubo-ovarian abscess (TOA) and may progress to peritonitis and Fitz-Hugh−Curtis syndrome (perihepatitis; see the image below). [1] Note that a rare but life-threatening complication of acute rupture of a TOA may result in diffuse peritonitis and necessitate urgent abdominal surgery. [2, 3, 4, 5]
Laparoscopy is the current criterion standard for the diagnosis of PID. No single laboratory test is highly specific or sensitive for the disease, but studies that can be used to support the diagnosis include the erythrocyte sedimentation rate (ESR), the C-reactive protein (CRP) level, and chlamydial and gonococcal DNA probes and cultures, endometrial biopsy, imaging studies (eg, ultrasonography, computed tomography [CT], and magnetic resonance imaging [MRI]) may be helpful in unclear cases. (See Workup.)
Management of pelvic inflammatory disease
Most patients with PID are treated in an outpatient setting. In selected cases, however, physicians should consider hospitalization. (See Treatment.)
Empirical antibiotic treatment is recommended for patients with otherwise unexplained uterine or adnexal tenderness and cervical motion tenderness, according to guidelines from the Centers for Disease Control and Prevention (CDC). [6] Antibiotic regimens for PID must be effective against Chlamydia trachomatis and Neisseria gonorrhoeae, as well as against gram-negative facultative organisms, anaerobes, and streptococci. (See Treatment and Medication.)
Background
PID is initiated by infection that ascends from the vagina and cervix into the upper genital tract. Chlamydia trachomatis is the predominant sexually transmitted organism associated with PID. Of all acute PID cases, less than 50% test positive for the sexually transmitted organisms such as C trachomatis and N gonorrhoeae. [7]
Other organisms implicated in the pathogenesis of PID include Gardnerella vaginalis (which causes bacterial vaginosis [BV]), Haemophilus influenzae, and anaerobes such as Peptococcus and Bacteroides species. Laparoscopic studies have shown that in 30-40% of cases, PID is polymicrobial. (See Etiology.)
Pathophysiology
Most cases of PID are presumed to occur in 2 stages. The first stage is acquisition of a vaginal or cervical infection. This infection is often sexually transmitted and may be asymptomatic. The second stage is direct ascent of microorganisms from the vagina or cervix to the upper genital tract, with infection and inflammation of these structures.
The mechanism (or mechanisms) by which microorganisms ascend from the lower genital tract is unclear. Studies suggest that multiple factors may be involved. Although cervical mucus provides a functional barrier against upward spread, the efficacy of this barrier may be decreased by vaginal inflammation and by hormonal changes that occur during ovulation and menstruation.
In addition, antibiotic treatment of sexually transmitted infections can disrupt the balance of endogenous flora in the lower genital tract, causing normally nonpathogenic organisms to overgrow and ascend. Opening of the cervix during menstruation, along with retrograde menstrual flow, may also facilitate ascent of microorganisms.
Intercourse may contribute to the ascent of infection through rhythmic uterine contractions occurring during orgasm. Bacteria may also be carried along with sperm into the uterus and fallopian tubes. [8]
In the upper genital tract, a number of microbial and host factors appear to influence the degree of inflammation that occurs and, thus, the amount of subsequent scarring that develops. Infection of the fallopian tubes initially affects the mucosa, but inflammation may rapidly become transmural. This inflammation, which appears to be mediated by complement, may increase in intensity with subsequent infections.
Inflammation may extend to uninfected parametrial structures, including the bowel. Infection may extend via spillage of purulent materials from the fallopian tubes or via lymphatic spread beyond the pelvis to produce acute peritonitis and acute perihepatitis (Fitz-Hugh−Curtis syndrome).
Pregnancy-related factors
PID rarely occurs in pregnancy; however, chorioamnionitis can occur in the first 12 weeks of gestation, before the mucous plug solidifies and seals off the uterus from ascending bacteria. Fetal loss may result. Concurrent pregnancy influences the choice of antibiotic therapy for PID and demands that an alternative diagnosis of ectopic pregnancy be excluded. Uterine infection is usually limited to the endometrium but may be more invasive in a gravid or postpartum uterus.
A systematic review by Marcinkowski et al identified the following risk factors for PID during pregnancy [9] :
-
Presence of maternal pelvic structural anomalies
-
A history of sexually transmitted infections
-
Recent pelvic surgery
-
In vitro fertilization or oocyte retrieval
Genetic factors
Genetically mediated variation in immune response plays an important role in susceptibility to PID. [10] Variants in the genes that regulate toll-like receptors (TLRs), an important component in the innate immune system, have been associated with an increased progression of C trachomatis infection to PID. [11]
Den Hartog et al found a possible contributing role of 5 single-nucleoside polymorphisms (SNPs) in 4 genes encoding pattern recognition receptors in local tubal cells and circulating immune cells (eg, macrophages). The presence of 2 or more SNPs appeared to correlate with increased laparoscopically identifiable tubal pathology. [12]
Etiology
The organisms most commonly isolated in cases of acute PID are N gonorrhoeae and C trachomatis. [13] C trachomatis is an intracellular bacterial pathogen and the predominant sexually transmitted organism that causes PID.
In the United States, N gonorrhoeae is no longer the primary organism associated with PID, but gonorrhea remains the second most frequently reported sexually transmitted disease, after chlamydial infection. Clinically, gonorrheal infection may be asymptomatic or may manifest similarly to chlamydial infection; however, it more often produces more acute symptomatic disease. An estimated 10-20% of untreated chlamydial or gonorrheal infections progress to PID. [14, 15, 16]
Cultures of specimens collected during laparoscopy have demonstrated that PID is a polymicrobial infection in as many as 30-40% of cases. Polymicrobial PID may begin as an isolated infection with N gonorrhoeae or C trachomatis, which causes inflammation of the upper genital tract that facilitates the involvement of other pathogens (anaerobes, facultative anaerobes, and other bacteria). These other organisms are increasingly isolated as inflammation increases and abscesses form.
In addition to N gonorrhoeae and C trachomatis, [17] organisms involved in PID include the following:
-
Mycoplasma hominis
-
Ureaplasma urealyticum
-
Herpes simplex virus 2 (HSV-2)
-
Trichomonas vaginalis
-
Cytomegalovirus (CMV)
-
Haemophilus influenzae
-
Streptococcus agalactiae
-
Enteric gram-negative rods (eg, Escherichia coli)
-
Enterococcus, described in 2 individuals post IUD insertion [20]
-
Peptococcus species
-
Anaerobes
The microbiology of PID reflects the predominant sexually transmitted pathogens within a specific population, as well as some organisms less commonly seen in that population. Bacterial vaginosis (BV) may lead to vaginal inflammation, which could facilitate ascending infection with BV-associated organisms (eg, G vaginalis). However evidence is unclear if detecting and treating BV reduces PID-related infection. [21] In some regions, PID may be from a granulomatous salpingitis caused by Mycobacterium tuberculosis or Schistosoma species. [22]
In a cross-sectional study of 736 women with PID, patients with Trichomonas infections demonstrated a 4-fold increase in the histologic evidence of acute endometritis. Coinfection with HSV-2, N gonorrhoeae, C trachomatis, and BV were associated with histologic evidence of acute endometritis. HSV-2 was associated with fallopian tube inflammation and lower tract ulcerations that may contribute to disruption of the endocervical canal mucous barrier. [23]
HIV infection is associated with an increased incidence of infection with C trachomatis, Candida, and human papillomavirus (HPV). N gonorrhoeae can facilitate HIV transmission via modulation of HIV-specific immune responses. [24] Women with HIV infection also have an increased risk of progression to PID and TOA. [25]
Microbial virulence appears to play a significant role in PID. Bjartling et al studied different chlamydial strains recovered from patients with PID and found less symptomatic disease in infection produced by a less virulent variant strain. [26] Features that may increase the likelihood that a lower tract infection will progress to frank PID include expression of chlamydial heat shock protein 60 (CHSP60) in C trachomatis [27] and expression of P9Opa(b) protein in N gonorrhoeae. [28]
A study by Haggerty et al found an association between PID and bacterial vaginosis-associated bacteria. The four new species detected were Sneathia (Leptotrichia) sanguinegens, S amnionii, Atopobium vaginae, and BV-associated bacteria 1. [29, 30]
A study by Mitchell et al reported that fewer than half of patients with a diagnosis of PID have gonococcal or chlamydial infection. Mycoplasma genitalium, respiratory and gastrointestinal pathogens, and bacteria associated with bacterial vaginosis may account for a considerable number of PID cases. [31]
Risk factors
Risk factors for PID include multiple sexual partners, a history of prior STIs, and a history of sexual abuse. [32] Frequent vaginal douching has been considered a risk factor for PID, [33] but studies reveal no clear association. [34] Gynecologic surgical procedures which affect the cervix such as endometrial biopsy, curettage (after termination of pregnancy), and hysteroscopy break the cervical barrier, predisposing women to ascending infections. [35, 36]
Younger age has been found to be associated with an increased risk of PID. Likely reasons include increased cervical mucosal permeability, a larger zone of cervical ectopy, a lower prevalence of protective antichlamydial antibodies, and increased risk-taking behaviors. Older women with PID are more likely to be affected with non STI organisms.
Contraception
Different forms of contraception may affect the incidence and severity of PID. Appropriately used barrier contraception has clearly been shown to decrease the acquisition of most STIs. [37]
Studies of oral contraceptive pills (OCPs) have found differing effects on PID risks. On one hand, some authors suggest that OCPs increase the risk of endocervical infection, probably by increasing the zone of cervical ectopy. On the other hand, some evidence indicates that OCPs can decrease the risk of symptomatic PID, possibly by increasing cervical mucus viscosity, decreasing menstrual anterograde and retrograde flow, and modifying local immune responses. Still other studies have suggested that OCPs may not have any effect on PID incidence. [37]
Use of an intrauterine device (IUD) has been linked to a 2- to 9-fold increased risk of PID, but current IUDs may pose a substantially lower risk. [38] In a large retrospective cohort study from 2012, the overall risk of PID in women receiving IUDs was 0.54%. [39]
Kelly et al reported 9.6 cases of PID per 1000 IUD insertions, with the most significant risk in the first 20 days. [40] Meirik et al validated the risk of PID within the first month after insertion and also found that the risk appears to be modified by the patient’s number of sexual partners and age and by the community prevalence of STIs. [41] The CDC notes that the risk of PID is greatly reduced by testing for—and, if necessary, treating—STI before IUD insertion. [42, 43] This testing can be completed in the same day as insertion and patients confirmed to have STI’s should be treated. Additionally, IUDs need not be removed if PID is detected. Patients should be treated and re-evaluated clinically. If pain persists or symptoms are not improving, that is an indication to remove the IUD. [42]
PID may have a different microbial profile in IUD users. Viberga et al found that in women with PID, Fusobacterium and Peptostreptococcus species were significantly more common in IUD users than in non-IUD users. Actinomyces species were found almost exclusively in patients with IUDs. [44]
Bilateral tubal ligation (BTL) has not been found to provide protection against PID. However, patients with BTL may have delayed or milder forms of PID. [45]
Epidemiology
United States statistics
Among 1171 sexually experienced reproductive-aged women in the 2013-2014 National Health and Nutrition Education Survey (NHANES) the prevalence of self-reported lifetime PID was 4.4%. Therefore, approximately 2.5 million women aged 18-44 years nationwide have received a diagnosis of PID in their lifetime (95% CI = 1.8-3.2 million). [21] In a follow-up study that used the NHANES data, Kreisel et al reported that the prevalence of self-reported PID was highest among non-Hispanic Black women and women who lived in the South. [46]
The CDC has estimated that more than 1 million women experience an episode of PID every year. The disease leads to approximately 2.5 million office visits and 125,000-150,000 hospitalizations yearly. [47, 48]
International statistics
No specific international data are available for PID incidence worldwide. In 2005, however, the World Health Organization (WHO) estimated that approximately 448 million new cases of curable STIs occur annually in individuals aged 15-49 years. [49] Factors contributing to the difficulty of determining the actual worldwide incidence and prevalence of PID include the following [50] :
-
Nonrecognition of disease on the part of patients
-
Difficulties in obtaining access to care
-
The often subjective method of disease diagnosis
-
The lack of diagnostics and laboratory facilities in many developing countries
-
Underfunded and overstretched public health systems
Worldwide, WHO has determined that STIs rank in the top 5 disease categories for which adults seek care. Women in resource-poor countries, especially those in sub-Saharan Africa and Southeast Asia, experience an increased rate of complications and sequelae.
The annual rate of PID in high-income countries has been reported to be as high as 10-20 per 1000 women of reproductive age. Public health efforts implemented in Scandinavia to decrease the prevalence of STIs have been quite effective in reducing the incidence of PID. [51, 52]
Prognosis
PID has 3 principal complications, as follows:
-
Chronic pelvic pain
-
Infertility
-
Ectopic pregnancy
Chronic pelvic pain occurs in approximately 25% of patients with a history of PID. This pain is thought to be related to cyclic menstrual changes, but it also may be the result of adhesions or hydrosalpinx.
Impaired fertility is a major concern in women with a history of PID. Infection and inflammation can lead to scarring and adhesions within tubal lumens. Of women with tubal factor infertility, 50% have no history of PID but have scarring of the fallopian tubes and exhibit antibodies to C trachomatis. The rate of infertility increases with the number of episodes of infection. These sequelae help guide the CDC recommendation and United States Preventative Services Task Force (USPSTF) to annually screen sexually active females under age 25 or those older than age 25 who are high risk for C. trachomatis. [53] The risk of ectopic pregnancy is increased 15-50% in women with a history of PID. Ectopic pregnancy is a direct result of damage to the fallopian tube.
PID may produce TOA and extend to produce pelvic peritonitis and Fitz-Hugh−Curtis syndrome (perihepatitis). [54, 55] TOA is reported in as many as one third of women hospitalized for PID. Acute rupture of a TOA with resultant diffuse peritonitis is a rare but life-threatening event that calls for urgent abdominal surgery. [2, 3, 4, 5]
Approximately 125,000-150,000 hospitalizations occur yearly in the United States because of PID. [47] Women in resource-poor countries, especially those in sub-Saharan Africa and Southeast Asia, experience an increased rate of complications and sequelae; reasons for these higher rates include lack of access to care and inability to afford optimal care.
Studies of Taiwanese databases that included more than 60,000 women diagnosed with PID found that PID was an independent risk factor for myocardial infarction in patients older than 55 years [56] and that risk of stroke was increased in the 3 years following PID. [57] Another large-scale study from Taiwan found that the risk of ovarian cancer is also increased, particularly in women who have had at least 5 episodes of PID. [58]
A study by Trabert et al that included two independent populations reported that antibodies against prior or current C trachomatis (Pgp3) doubled the risk for ovarian cancer. [59] A Swedish study by Jonsson et al found that patients with a history of PID are at greater risk for epithelial ovarian cancer and that the risk increases with the number of PID episodes. [60]
Patient Education
Patient education should focus on methods of preventing PID and STIs, including reducing the number of sexual partners, avoiding unsafe sexual practices, and routinely using appropriate barrier protection. Adolescents are at increased risk for PID and should therefore be advised to delay the onset of sexual activity until age 16 years or older. [61]
After treatment, women should be counseled to abstain from sexual activity or educated to use barrier protection strictly and appropriately until their symptoms have fully abated and they have completed their antibiotic regimen. The woman’s sexual partner should also be treated for STI if necessary.
For patient education information, see the Women’s Health Center, Sexual Health Center, and Pregnancy Center, as well as Pelvic Inflammatory Disease, Ectopic Pregnancy, Birth Control Overview, Birth Control Methods, and Female Sexual Problems.
-
"Violin-string" adhesions of chronic Fitz-Hugh-Curtis syndrome.
-
Transabdominal ultrasonogram shows anechoic tubular structures in adnexa; finding is compatible with hydrosalpinx.
-
Endovaginal ultrasonogram reveals tubular structure with debris in left adnexa; finding is compatible with pyosalpinx.
-
Ultrasonogram shows markedly heterogeneous and thickened endometrium; finding is compatible with endometritis.
-
Ultrasonogram reveals bilateral complex masses in patient who had pyometrium; finding is compatible with tubo-ovarian abscess.
-
Transabdominal ultrasonogram demonstrates echogenic region within endometrium with dirty shadowing; finding is compatible with air in endometrium and endometritis. Additionally, bilateral complex masses are present; finding is compatible with tubo-ovarian masses.