Practice Essentials
Meningioma refers to a set of tumors that arise contiguously to the meninges (see the image below). Meningiomas may occur intracranially or within the spinal canal. They are thought to arise from arachnoidal cap cells, which reside in the arachnoid layer covering the surface of the brain.
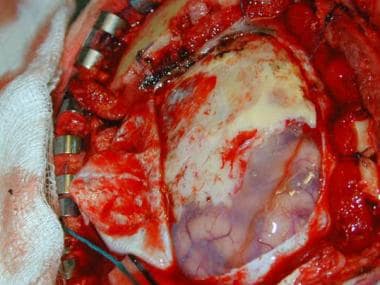 Case 1: Surgical view of the tumor. The dura is opened, and the meningioma can be seen extending en plaque over the surface of the brain.
Case 1: Surgical view of the tumor. The dura is opened, and the meningioma can be seen extending en plaque over the surface of the brain.
Signs and symptoms
Meningiomas produce their symptoms by several mechanisms. They may cause symptoms by irritating the underlying cortex, compressing the brain or the cranial nerves, producing hyperostosis [1] and/or invading the overlying soft tissues, or inducing vascular injuries to the brain. [2]
Diagnosis
Imaging studies are the mainstay of diagnosis. Plain skull radiograph may reveal hyperostosis and increased vascular markings of the skull, as well as intracranial calcifications. On plain head CT scans, meningiomas are usually dural-based tumors that are isoattenuating to slightly hyperattenuating.
Management
In general, the ideal treatment of a benign meningioma is surgical resection if possible.
Stereotactic radiosurgery has been shown to provide excellent local tumor control with minimal toxicity.
Background
Meningioma, the term coined by Harvey Cushing, refers to a set of tumors that arise contiguously to the meninges (see the image below).
 Case 1: Surgical view of the tumor. The dura is opened, and the meningioma can be seen extending en plaque over the surface of the brain.
Case 1: Surgical view of the tumor. The dura is opened, and the meningioma can be seen extending en plaque over the surface of the brain.
Pathophysiology
Meningiomas may occur intracranially or within the spinal canal. They are thought to arise from arachnoidal cap cells, which reside in the arachnoid layer covering the surface of the brain. See the images below.
Meningiomas commonly are found at the surface of the brain, either over the convexity or at the skull base. In rare cases, meningiomas occur in an intraventricular or intraosseous location. The problem of classifying meningioma is that arachnoidal cells may express both mesenchymal and epithelial characteristics. Other mesodermal structures also may give rise to similar tumors (eg, hemangiopericytomas or sarcomas). The classification of all of these tumors together is controversial. The current trend is to separate unequivocal meningiomas from other less well-defined neoplasms. Undoubtedly, advances in molecular biology will allow scientists to determine the exact genomic aberration responsible for each specific neoplasm.
Epidemiology
Frequency
United States
The annual incidence of symptomatic meningiomas is approximately 2 cases per 100,000 individuals. Meningiomas account for approximately 20% of all primary intracranial neoplasms. However, the true prevalence is likely higher than this because autopsy studies reveal that 2.3% of individuals have undiagnosed asymptomatic meningiomas. Meningiomas are multiple in 5-40% of cases, particularly when they associated with neurofibromatosis type 2 (NF2). Familial meningiomas are rare unless associated with NF2. [5]
International
The frequency of meningiomas in Africa is nearly 30% of all primary intracranial tumors. [6]
Mortality/Morbidity
Mortality and morbidity rates for meningiomas are difficult to assess. Some meningiomas are discovered fortuitously when CT or MRI is done to assess for unrelated diseases or conditions. Therefore, some patients die with meningioma and not from it. Estimates of the 5-year survival usually range from 73-94%.
A systematic review of the literature regarding the clinical behavior of small, untreated meningiomas suggests that most meningiomas 2.5 cm or less in diameter do not proceed to cause symptoms in the 5 years following their discovery. Patients with tumors 2.5-3 cm in initial size went on to develop new or worsened symptoms 17% of the time. Those that do cause symptoms can usually be predicted with close radiographic follow-up. [7]
Meningiomas usually grow slowly, and they may produce severe morbidity before causing death.
Factors that may be predictive of a high postoperative morbidity rate include patient-related factors (eg, advanced age, comorbid states such as diabetes or coronary artery disease, preoperative neurological status), tumor factors (eg, location, size, consistency, vascularity, vascular or neural involvement), previous surgery, or previous radiation therapy.
Race
Meningiomas are more prevalent in Africa than in North America or Europe. In Los Angeles County, meningioma is reported more commonly in African Americans than in others.
Sex
Meningiomas afflict women more often than men. The male-to-female ratio ranges from 1:1.4 to 1:2.8.
-
The female preponderance may be less pronounced in the black population than in other groups.
-
Meningiomas are equally distributed between boys and girls.
Age
The incidence increases with age. Ages and corresponding incidence rates reported from 2002 are as follows:
-
Age 0-19 years - 0.12
-
Age 20-34 years - 0.74
-
Age 35-44 years - 2.62
-
Age 45-54 years - 4.89
-
Age 55-64 years - 7.89
-
Age 65-74 years - 12.79
-
Age 75-84 years - 17.04
-
Age 85 years and older - 18.86
Prognosis
Patients whose meningiomas are completely resected usually have an excellent prognosis.
Tumor size may play a role in determining outcome. In a study of 34 patients who underwent surgery for CPA meningiomas, Agarwal et al found that the rate of permanent cranial nerve deficits was significantly greater in patients with tumors of more than 3 cm in size than in those with smaller meningiomas (45.5% vs 5.9%, respectively). It was also found that deficits of the lower cranial nerves occurred only in patients whose tumors extended into the jugular foramen. No association was found between tumor extension into the internal acoustic canal and either postoperative complications or cranial nerve deficits. Among all patients, 5.9% suffered postoperative facial nerve palsy. [8]
The following types of meningiomas are most likely to recur: incompletely excised, malignant, or multiple tumors.
-
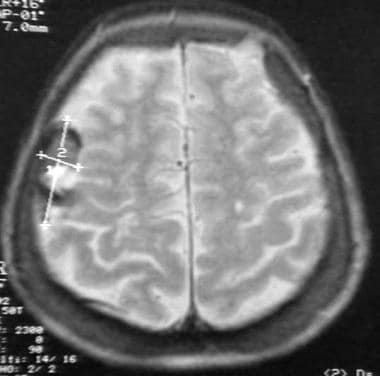
Case 1: MRI of a meningioma on plaque.
-
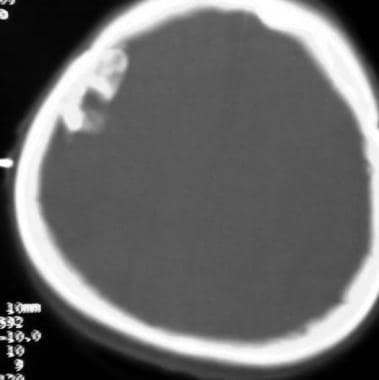
Case 1: Bone-window CT reveals calcification of the meningioma.
-
Case 1: Surgical view of the tumor. The dura is opened, and the meningioma can be seen extending en plaque over the surface of the brain.
-
Case 1: Bone flap seen along the removed meningioma in toto.
-
Case 2: Gadolinium-enhanced MRI of a meningioma invading the overlying dura and bone. Compare with appearance in Case 1.
-
Case 2: Bone-window CT scan reveals the skull involvement. Note the absence of tumoral calcification.
-
Case 2: Intraoperative view shows the skull involvement.
-
Case 2: Bone flap was removed. Note tumoral breach of the dura. The dura and overlying skull were removed surgically. Duraplasty and cranioplasty were performed
-
Case 2: Surgical specimen. Complete resection was achieved.
-
Case 3: Tentorial meningioma. A, Contrast-enhanced CT scan shows the enhancing meningioma. Transverse T1-weighted MRIs shows isointensity of the tumor compared with the surrounding brain (B) and its homogenous enhancement (C). Coronal (D), coronal enhanced (E), and sagittal enhanced (F) T1-weighted MRIs. Posterior circulation angiograms show tumoral blush (arrow in G) and the Bernasconi-Cassinari artery (arrow in H).
-
Case 3: Tentorial meningioma. Gadolinium-enhanced T1-weighted MRI immediately (A) and 2 years after surgery (B-D). Transverse images show posterior (arrow in B) and anterior (arrow in C) recurrence involving the tentorium. Sagittal images show posterior (D) and anterior (E) recurrence involving the tentorium. Lower vignette reveals complete excision of the recurrence after a second operation.
-
Case 3: Tentorial meningioma A, Pathology showed syncytial meningioma. Note hypercellularity and minimal whorling (hematoxylin-eosin, original magnification X400). B, MRI performed 4 years after the first operation reveals a recurrence over the posterior tentorium. C, Two-dimensional planning for stereotactic radiosurgery. Three recurrences lie in the plane of the tentorium on a single line. D, Three-dimensional planning for stereotactic radiosurgery. Three arcs were used to irradiate the largest recurrence.
-
Case 4: Recurrent subcutaneous meningioma. A, Patient underwent surgery for a parieto-occipital meningioma in 1978. She was lost to follow-up until 1996, when this transverse T2-weighted MRI was obtained. Arrow indicates surgical bed of the resected meningioma. B, Although the initial surgical bed is tumor-free, sagittal T2-weighted MRI shows a large subcutaneous recurrence. C, Lower transverse section also shows recurrence. Note variegated appearance of the tumor. D, Transverse section at a lower level. Postoperative sagittal (E) and transverse (F, G) enhanced T1-weighted MRI shows gross total removal of the tumor. H and I, Tumoral recurrence 3 months after surgery, at the same level as in G and F, respectively. Patient received repeat surgery for subtotal removal of the tumor; a pediculated subcutaneous flap was used to close the surgical defect. After surgery, patient received conventional radiotherapy.
-
Case 5: Bilateral olfactory meningioma invading the facial sinuses. Coronal (A), transverse (B), and sagittal (C) gadolinium-enhanced T1-weighted MRI shows bilateral olfactory meningiomas, and the falx dividing the tumor in 2. Arrow indicates tumor invasion of the sinuses. D, Postoperative enhanced T1-weighted MRI shows that the tumor was completely removed by means of craniotomy and a transfacial approach. E, Tumor was first approached intracranially. Enhanced T1-weighted MRI reveals complete excision of the intracranial component. Arrow indicates residual in the sinuses. F, Residual was completely excised by means a transfacial approach performed with the otolaryngology team.
-
Case 6: Subfrontal meningioma in a patient with abnormal behavior. A, Contrast-enhanced CT scan clearly shows bilateral subfrontal meningioma. B, Transverse T1-weighted MRI of same lesion. C, Intense gadolinium enhancement of the tumor. Coronal (D) and sagittal (E) gadolinium-enhanced T1-weighted MRIs. F, Anterior circulation angiogram reveals posterior displacement of the anterior cerebral artery by tumor. G, Postoperative MRI shows complete removal of the tumor. H-I, Pathology slides (hematoxylin-eosin; original magnification X100 in H, X400 in I) show syncytial meningioma with well-identified whorls and no psammoma bodies.
-
Case 7: Parasagittal meningioma invading the superior sagittal sinus (SSS). A, Sagittal T1-weighted MRI shows a meningioma (arrow). B, T2-weighted MRI. Note midline shift and tumoral invasion of the skull (arrow). C, Transverse T2-weighted MRI. D, Angiogram shows invasion of the SSS, which remains patent. Sagittal (E, G), transverse (F) postoperative T1-weighted MRI. H, Gadolinium-enhanced postoperative T1-weighted MRI shows residual tumor, which was intentionally left to preserve patency of the SSS. I, Pathology slide (hematoxylin-eosin, original magnification X100) shows a highly vascular syncytial meningioma.
-
Pathology slides (hematoxylin-eosin; original magnification X400 in A-B, X100 in C-D). A, Fibroblastic meningioma (arrowheads) abutting the dura (arrow). B, Psammomatous meningioma (arrow indicates psammoma body). C, Meningothelial meningioma, tumor in case 4. E, Meningioma with marked vascularity (arrowheads indicate meningioma cluster; arrow, vessel wall).
-
Case 4: Pathology slides (hematoxylin-eosin, original magnification X400). A, Meningioma with malignant features, as evinced by prominent nucleoli (yellow dot) and mitoses (arrows). B, Intranuclear cytoplasmic intrusion (pseudoinclusion).
-
This is an extra-axial tumor. Glioblastoma multiforme (GBM) and astrocytoma are intraparenchymal tumors, and GBM enhances in a variegated fashion. Acoustic schwannomas are seen in the posterior fossa but not in this location. Fibrous dysplasia involves the skull but does not cause this amount of compression.
-
Surgery on a 46-year-old female with a 2-cm, dural-based enhancing tumor along the left frontal convexity. The lesion was presumed to be a meningioma and showed serial enlargement on MRI, prompting the procedure. Pathology confirmed the tumor to be a WHO grade I meningioma. Video courtesy of Anand I. Rughani, MD, and Jeffrey E. Florman, MD.
-
Meningioma resection in the tuberculum sellae. Video courtesy of Anand I. Rughani, MD, and Jeffrey E. Florman, MD.