Background
Paraneoplastic syndromes are a group of rare disorders that are triggered by an abnormal immune system response to an underlying malignant tumor. Patients with paraneoplastic neurological syndrome (PNS) most often present with neurologic symptoms before an underlying tumor is detected or coincide with the cancer diagnosis.
Paraneoplastic neurologic syndromes include a variety of neurologic disorders, such as paraneoplastic cerebellar degeneration (PCD), that are caused by an immune-mediated mechanism rather than a metastatic complication or medication effect. Any malignancy can cause a paraneoplastic syndrome and any part of the nervous system can be involved depending on the type of primary malignancy. These syndromes affect 1–3% of all cancer patients. [1] These syndromes are difficult to diagnose and typically respond poorly to treatment. However, the oncologic outcome of patients with antibody-associated paraneoplastic syndromes does not significantly differ from that of patients who do not have the antibodies or a paraneoplastic syndrome.
PCD is a rare nonmetastatic complication of a carcinoma, typically mediated by antibodies generated against tumor antigens (proteins). Similar proteins are also expressed on Purkinje cells and possibly other cells within the cerebellum. The cancer-fighting antibodies mistakenly attack these normal protein cells in the cerebellum. This immune activation in the central nervous system (CNS) results in cerebellar injury and dysfunction. [2, 3]
An association between PCD and occult gynecologic cancers (breast or ovarian) was first identified in 1938, and the syndrome was described fully by Brain in 1951. [4] Posner found that patients with PCD can be classified according to the presence or absence of an antibody that reacted with an antigen present in both the tumors and in cerebellar Purkinje neurons obtained from these patients. [5]
PCD is a syndrome that occurs predominantly in patients with cancer of the ovary, uterus, or adnexa; cancer of the breast; small-cell carcinoma of the lung; or Hodgkin lymphoma. [6, 7]
The onset of symptoms of cerebellar degeneration indicates the presence of an occult malignancy. Not all gynecologic cancers present as paraneoplastic neurologic syndrome; however, in a clinical presentation consistent with a paraneoplastic neurologic syndrome, the chances of underlying malignancy are very high.
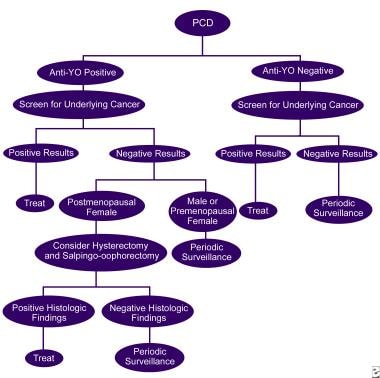
The image below illustrates the workup of PCD.
Diagnostic criteria
The 2021 updated diagnostic criteria for paraneoplastic neurologic syndromes (PNS), which include PCD as one of the more common presentations, provide a refined approach to diagnosing PNS by incorporating new insights into the phenotypes and associated antibodies. [8, 2] The key aspects of the updated criteria include:
Classification of phenotypes
The criteria substitute the older term "classical syndromes" with "high-risk phenotypes" for cancer and introduce "intermediate-risk phenotypes" to capture a broader spectrum of clinical presentations.
Risk categorization of antibodies
Antibodies are now categorized into "high-risk" (more than 70% associated with cancer) and "intermediate-risk" (30%–70% associated with cancer). This categorization helps in determining the likelihood of a paraneoplastic origin.
Levels of evidence
The criteria define three levels of evidence for diagnosing PNS: definite, probable, and possible. The level is determined by a scoring system known as the PNS-Care Score, which considers the clinical phenotype, type of antibody detected, presence or absence of cancer, and follow-up duration.
Diagnostic pathway
A diagnosis of definite PNS generally requires the presence of high- or intermediate-risk antibodies, except in the case of opsoclonus-myoclonus syndrome, which has separate considerations.
Pathophysiology
Paraneoplastic cerebellar degeneration (PCD) is caused by the secondary effects of cancer and is believed to be immune mediated [2, 3] High titers in the patient's serum and cerebrospinal fluid (CSF) of autoantibodies directed against both neurons and tumor have been detected in some forms of this syndrome. [9, 10] These autoantibodies are considered the result of an immunologic response to tumor and may cross-react with cells of the nervous system, causing neuronal damage.
Specific forms of this syndrome often are associated with specific antineuronal antibodies and tumors. The onset of neurologic symptoms and detection of these antibodies precede diagnosis of the tumor more 60% of the time. Therefore, detection of these antibodies greatly assists the diagnosis of this syndrome and prompts investigations for the underlying tumor. [2] Not all patients presenting with PCD and its clinical features have recognizable antineuronal antibodies. However, this does not exclude the likelihood of occult malignancy. [11, 12] In approximately 40% of patients, no antibodies are identified. Although any paraneoplastic antibody may cause PCD, only anti-Yo, anti-Tr, and antimetabotropic glutamate receptor 1 (mGluR1) have been shown to have specific association with isolated cerebellar dysfunction.
The Yo antigen is a cytoplasmic protein (CDR2) that interacts with c-Myc. CDR2 is expressed mostly on the Purkinje cells of the cerebellum and can also be present in neurons of the brain stem. Studies suggest that CDR2 sequesters c-Myc in the neuronal cytoplasm and downregulates its activity. Disruption of this interaction by anti-Yo antibodies may increase c-Myc activity, leading to apoptosis of the Purkinje cells. [13, 14] The finding of gliosis and near total loss of Purkinje cells on autopsies of patients with anti-Yo antibodies supports this theory. [15]
In cases with associated Lambert-Eaton myasthenic syndrome, anti-voltage-gated calcium channel (anti-VGCC) antibodies mediate auto-immunity against P/Q type VGCC, which are membrane proteins on Purkinje cells that drive the activity and survival of neurons. Thus, some patients with anti-VGCC antibody have been found to have diffuse loss of Purkinje cells, causing cerebellar degeneration. [16]
Antibodies could therefore play an initial pathogenic role in PCD, but the T-cell immune response is believed to be the major effector of neuronal degeneration.41 [3] As a result, patients with PCD have been shown to have infiltration of CD8+ T cells in the cerebellum, with cytotoxic T cells appearing proximal to damaged neurons. [15]
Epidemiology
Frequency
In one study, paraneoplastic cerebellar degeneration (PCD) was observed in 25% of paraneoplastic neurologic syndromes, occurring in 2 of every 1000 patients with cancer. [17]
In a large-scale UK study of 1500 patients with progressive cerebellar ataxia, 3% were found to have PCD. [18]
Mortality and morbidity
In the study cited above, median survival duration was 100 months for patients with breast cancer and 22 months for those with gynecologic cancer. Although paraneoplastic cerebellar degeneration led to the diagnosis of cancer in 63% of patients, cancer progression was the cause of death in 52%. [17]
Demogrpahics
Both sexes are affected, but PCD is far more common in women than in men.
PCD associated with anti-Yo antibody occurs in middle-aged women with occult ovarian or breast cancer that is usually indolent.
PCD associated with anti-Hu antibody occurs in middle-aged men and women or patients with risk factors for lung cancer.
When the condition is associated with Hodgkin lymphoma, patients are usually young men, and the cerebellar disease often follows the diagnosis of lymphoma.
Prognosis
Prognosis in paraneoplastic cerebellar degeneration (PCD) greatly depends on early detection of the underlying neoplasm and its stage at the time of detection.
In most cases, prognosis is poor. There tends to be little response to further antineoplastic or immunotherapy after tumor resecton. particulary in patients with anti-Yo and anti-Hu antibodies. [19] Those with other antibodies, such as anti-Tr, may have a better chance of responding to treatment.
-
The workup of paraneoplastic cerebellar degeneration.
-
MRI of a 29-year-old female with ARCA1. Sagittal T1 shows marked diffuse cerebellar atrophy with no atrophy of the cerebral cortex, midbrain, pons, or medulla. Image from National Institutes of Health.