Practice Essentials
Meningitis is a condition characterized by inflammation in the meninges and subarachnoid space, which can be caused by infections, underlying medical conditions, or medication reactions. The severity and onset of symptoms can vary. [1, 2]
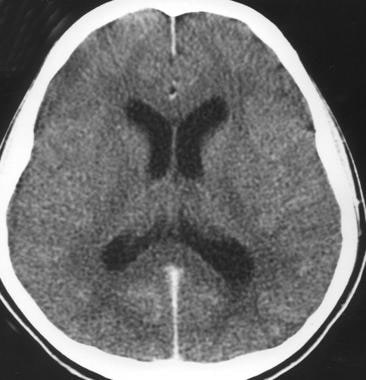 Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.
Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.
Signs and symptoms
The classical triad of bacterial meningitis consists of the following [1, 2] :
-
Fever
-
Headache
-
Nuchal rigidity (neck stiffness)
Less than half of patients have all three classical signs [3, 4] ; other symptoms can include nausea, vomiting, photalgia (photophobia), sleepiness, confusion, irritability, delirium, and coma. Patients with viral meningitis may have a history of preceding systemic symptoms (eg, myalgias, fatigue, or anorexia).
The history should address the following [1, 2, 5] :
-
Epidemiologic factors and predisposing risks such as mosquito bites (West Nile virus in endemic months, June-October in the United States)
-
Exposure to a sick contact (small children with febrile illness)
-
Previous medical treatment and existing conditions
-
Geographic location and travel history
-
Season and temperature (enterovirus and West Nile virus in the summer and fall; herpes simplex virus type 2 year round)
Acute bacterial meningitis in otherwise healthy patients who are not at the extremes of age presents in a clinically obvious fashion; however, subacute bacterial meningitis often poses a diagnostic challenge.
General physical findings in viral meningitis are common to all causative agents. Enteroviral infection is suggested by the following:
-
Exanthemas
-
Contact with small children with febrile illnesses
-
Symptoms of pericarditis, myocarditis, or conjunctivitis
-
Syndromes of pleurodynia, herpangina, and hand-foot-and-mouth disease
See Acute Pericarditis, Myocarditis, Viral Conjunctivitis, Pleurodynia, Herpangina, and Hand-foot-and-mouth Disease.
Infants may have the following:
-
Bulging fontanelle (if euvolemic)
-
Paradoxic irritability (ie, remaining quiet when stationary and crying when held)
-
High-pitched cry
-
Hypotonia
The examination should evaluate the following:
-
Focal neurologic signs
-
Signs of meningeal irritation
-
Systemic and extracranial findings
-
Level of consciousness
In chronic meningitis, it is essential to perform careful general, systemic, and neurologic examinations, looking especially for the following:
-
Lymphadenopathy
-
Papilledema
-
Meningismus
-
Cranial nerve palsies
-
Other focal neurologic signs
Patients with aseptic meningitis syndrome usually appear clinically nontoxic, with no vascular instability. They characteristically have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
See Clinical Presentation for more detail.
Diagnosis
The diagnostic challenges in patients with clinical findings of meningitis are as follows [1, 2, 6, 7] :
-
Early identification and treatment of patients with acute bacterial meningitis
-
Assessing whether a treatable CNS infection is present in those with suspected subacute or chronic meningitis
-
Identifying the causative organism
Blood studies that may be useful include the following [1, 7] :
-
Complete blood count (CBC) with differential
-
Serum electrolytes
-
Serum glucose (which is compared with the CSF glucose)
-
Blood urea nitrogen (BUN) or creatinine and liver profile
In addition, the following tests may be ordered [1, 7] :
-
Blood, nasopharynx, respiratory secretion, urine or skin lesion cultures or antigen/polymerase chain reaction (PCR) detection assays
-
Syphilis testing
-
Serum procalcitonin testing
-
Lumbar puncture and CSF analysis
-
Neuroimaging (CT of the head or MRI of the brain)
See Workup for more detail.
Management
Initial measures include the following [1] :
-
Shock or hypotension – Crystalloids
-
Altered mental status – Seizure precautions and treatment (if necessary), along with airway protection (if warranted)
-
Stable with normal vital signs – Oxygen, IV access, and rapid transport to the emergency department (ED)
Treatment of bacterial meningitis includes the following [1] :
-
Prompt initiation of empiric antibacterial therapy as appropriate for patient age and condition
-
After identification of the pathogen and determination of susceptibilities, targeted antibiotic therapy as appropriate for patient age and condition
-
Steroid (typically, dexamethasone) therapy
-
In certain patients, consideration of intrathecal antibiotics
The following systemic complications of acute bacterial meningitis must be treated [1] :
-
Hypotension or shock
-
Hypoxemia
-
Hyponatremia
-
Cardiac arrhythmias and ischemia
-
Stroke
-
Exacerbation of chronic diseases
Most cases of viral meningitis are benign and self-limited, but in certain instances, specific antiviral therapy may be indicated, if available.
Other types of meningitis are treated with specific therapy as appropriate for the causative pathogen, as follows [1] :
-
Fungal meningitis - Cryptococcal (amphotericin B, flucytosine, fluconazole), Coccidioides immitis (fluconazole, amphotericin B, itraconazole), Histoplasma capsulatum (liposomal amphotericin B, itraconazole), or Candida (amphotericin plus 5-flucytosine)
-
Tuberculous meningitis (isoniazid, rifampin, pyrazinamide, ethambutol, streptomycin)
-
Parasitic meningitis (amebic [Naegleria fowleri] or acanthamebic) - Variable regimens
-
Lyme meningitis (ceftriaxone; alternatively, penicillin G, doxycycline, chloramphenicol)
See Treatment and Medication for more detail.
Background
Infections of the central nervous system (CNS) can be categorized into two main groups: those that mainly affect the meninges (such as meningitis) and those that mainly affect the parenchyma (such as encephalitis). [1]
The 3 layers of membranes that enclose the brain and spinal cord. [1]
-
Dura mater - A hard outer membrane
-
Arachnoid mater - A lacy, weblike middle membrane
-
Pia mater – Is firmly attached to spinal cord and has rich blood supply
-
Subarachnoid space - A fragile fibrous inner layer that houses numerous blood vessels supplying the brain and spinal cord, located between the arachnoid mater and pia mater.
Arachnoid and pia mater are called leptomeninges
Meningitis is inflammation of leptomeninges including subarachnoid space leading to a constellation of signs and symptoms and presence of inflammatory cells in CSF.
Pachymeningitis is inflammation of dura mater that usually is manifested by thickening of the intracranial dura mater on radiology
Other definitions
-
Acute meningitis is defined as onset of symptoms of meningeal inflammation over the course of hours to several days [1]
-
Chronic meningitis is defined as at least 4 weeks of symptoms of inflammation of meninges [8]
-
Aseptic meningitis refers to a syndrome consistent with signs and symptoms of meningeal inflammation but with negative routine CSF cultures (See Aseptic Meningitis.)
-
Recurrent meningitis is defined as at least 2 episodes of signs and symptoms of meningeal inflammation with associated CSF findings separated by a period of full recovery. [9]
Pathophysiology
In most cases, meningitis is caused by an infectious agent that has colonized or established a localized infection in various parts of the body such as the skin, nose and throat, respiratory tract, gastrointestinal tract, or genitourinary tract. [1] The organism is able to invade the submucosa at these sites by bypassing the host's defenses (eg, physical barriers, local immunity, and phagocytes, or macrophages).
An infectious agent (such as a bacterium, virus, fungus, or parasite) can access the CNS and cause meningeal disease through any of the following three major pathways [1] :
-
Invasion of the bloodstream (eg, bacteremia, viremia, fungemia, or parasitemia) leading to subsequent hematogenous seeding of the CNS
-
Utilizing a retrograde neuronal pathway (eg, olfactory and peripheral nerves), as seen with organisms like Naegleria fowleri or Gnathostoma spinigerum
-
Direct contiguous spread through methods such as sinusitis, otitis media, congenital malformations, trauma, or direct inoculation during intracranial manipulation
Invasion of bloodstream, subsequent seeding
The most common mode of spread for many pathogens is through invasion of the bloodstream and subsequent seeding. [1] This pathway is characteristic of meningococcal, cryptococcal, syphilitic, and pneumococcal meningitis. On rare occasions, meningitis can result from invasion via septic thrombi or osteomyelitic erosion from infected neighboring structures. Meningeal seeding also can occur through direct bacterial inoculation during trauma, neurosurgery, or instrumentation. In newborns, meningitis can be transmitted vertically, involving pathogens that have colonized the maternal intestinal or genital tract, or horizontally, from nursery staff or caregivers at home.
Progressing from nearby infections such as otitis media, mastoiditis, or sinusitis, the expansion of bacteria into the brain's outer layers is a frequent occurrence. [1] The potential avenues for bacteria to travel from the middle ear to the meninges include the following:
-
The bloodstream
-
Existing tissue planes (eg, posterior fossa)
-
Fractures in the temporal bone
-
The oval or round window membranes within the inner ear's labyrinths
The protective barrier created by the meninges shields the brain from the immune system, but in cases of meningitis, this defense can be breached, enabling bacteria to infiltrate and cause infection. The body's effort to combat the infection may exacerbate the situation by causing blood vessels to become leaky, leading to brain swelling and diminished blood flow. [1] Severe bacterial meningitis can break through the pial barrier, causing extensive brain damage. The sustained inflammatory response in meningitis is fueled by factors like bacterial replication, increased inflammatory cells, and disruptions in membrane transport, resulting in alterations in the composition of cerebrospinal fluid, including changes in cell count, pH, lactate, protein, and glucose levels.
Exudates spread throughout the cerebrospinal fluid, primarily affecting the basal cisterns, leading to the following consequences [1] :
-
Cranial nerve damage (such as cranial nerve VIII, which can result in hearing loss)
-
Blockage of CSF flow (resulting in obstructive hydrocephalus)
-
Triggering of vasculitis and thrombophlebitis (resulting in localized brain ischemia)
Intracranial pressure and cerebral fluid
Meningitis can lead to increased intracranial pressure (ICP) due to various factors such as interstitial edema, cytotoxic edema, and vasogenic edema. [1] This can result from mechanisms like obstructed CSF flow, toxic factors released by bacteria and neutrophils, and increased permeability of the blood-brain barrier. Left untreated, the cycle of decreasing CSF, worsening cerebral edema, and rising ICP can continue, potentially causing complications like vasospasm, thrombosis, and systemic hypotension (septic shock) leading to systemic complications or diffuse central nervous system ischemic injury and ultimately, death.
Cerebral edema
The influx of plasma components into the subarachnoid space and impaired venous outflow contribute to increased cerebrospinal fluid (CSF) viscosity, leading to interstitial edema. [1] Bacterial byproducts, activated cells, and neutrophils lead to cytotoxic edema. This accumulation of fluids and cellular elements causes various types of cerebral edema, resulting in elevated intracranial pressure and reduced cerebral blood flow. Anaerobic metabolism, elevated lactate, and low glucose levels in the CSF may occur. Inadequately controlled, this process can lead to transient neuronal dysfunction or permanent injury if not effectively treated.
Cytokines and secondary mediators in bacterial meningitis
Advancements in understanding the pathophysiology of meningitis have shed light on the crucial roles of various cytokines (eg, tumor necrosis factor alpha [TNF-α] and interleukin [IL]-1), chemokines (IL-8), and proinflammatory molecules in pleocytosis and neuronal damage during bacterial meningitis episodes.
Patients with bacterial meningitis typically exhibit heightened levels of cytokines such as TNF-α, IL-1, IL-6, and IL-8 in their CSF. [1] These molecules are believed to play key roles in triggering the inflammatory cascade in meningitis through interactions with pattern-recognition receptors like Toll-like receptors (TLRs).
Among these cytokines, TNF-α and IL-1 are particularly notable for their involvement in the inflammatory process. [1] TNF-α, derived from cells like monocyte-macrophages and astrocytes, and IL-1, produced by activated mononuclear phagocytes, are prominently detected in the CSF of bacterial meningitis patients. Secondary mediators like IL-6, IL-8, nitric oxide, prostaglandins, and platelet activation factor are thought to amplify the inflammatory response synergistically or independently.
This cascade of events can lead to vascular endothelial injury, increased blood-brain barrier permeability, and the influx of blood components into the subarachnoid space. [1] Neutrophils then are attracted to the area, crossing the damaged blood-brain barrier and contributing to the pronounced neutrophilic pleocytosis seen in bacterial meningitis.
Genetic predisposition to inflammatory response
In cases of bacterial meningitis, the inflammatory response triggers the recruitment of an excessive number of neutrophils to the subarachnoid space. These activated neutrophils release harmful substances such as oxidants and metalloproteins, which can damage brain tissue.
Pattern recognition receptors, particularly TLR A4 (TLRA4), activate the MyD88-dependent pathway, leading to the overproduction of proinflammatory mediators. Dexamethasone is used to mitigate the cellular toxicity caused by neutrophils. Ongoing research is focused on developing strategies to inhibit TLRA4 and other proinflammatory receptors through genetically engineered suppressors. [1]
Etiology
Acute Bacterial Meningitis
Bacterial meningitis is characterized by a pyogenic inflammatory response in the meninges and subarachnoid cerebrospinal fluid (CSF), caused by bacterial infection. It typically presents with a sudden onset of meningeal symptoms and an increase in neutrophils in the CSF. Without prompt treatment, bacterial meningitis can result in lifelong disability or even death. [1, 10, 11] Depending on age and general condition, patients with acute bacterial meningitis present acutely with signs and symptoms of meningeal inflammation and systemic infection of less than 24 hours’ (and usually >12 hours’) duration.
Bacterial meningitis typically occurs when bacteria enter the meninges through the bloodstream, with colonization of the nasopharynx being a common source in cases where the infection is not clearly identified. Many bacteria that cause meningitis, such as Neisseria meningitidis and Streptococcus pneumoniae, often are present in the nose and throat without causing symptoms.
Certain respiratory viruses may weaken mucosal defenses, making it easier for bacterial agents to enter the bloodstream. Once in the blood, these pathogens must evade immune responses, including antibodies, complement-mediated bacterial killing, and neutrophil phagocytosis.
Subsequently, the bacteria can spread to different parts of the body, including the central nervous system (CNS). The specific mechanisms by which these infectious agents reach the subarachnoid space are not fully understood. Inside the CNS, the pathogens can thrive as immune defenses, such as immunoglobulins, neutrophils, and complement factors, are limited in this region. The uncontrolled presence and replication of these infectious agents can trigger the inflammatory cascade seen in meningitis.
The specific infectious agents that are involved in bacterial meningitis vary among different patient age groups, and the meningeal inflammation may evolve into the following conditions:
-
Ventriculitis
-
Empyema
-
Cerebritis
-
Abscess formation
Some of the bacteria associated with bacterial meningitis include the following [11, 12] :
Acinetobacter spp
Capnocytophaga canimorsus
Coagulase negative Staphylococcus
Cutibacterium acnes
Enterococcus spp
Escherichia coli
Fusobacterium necrophorum
Haemophilus influenzae
Klebsiella pneumoniae
Listeria monocytogens
Pasteurella multocida
Pseudomonas aeruginosa
Salmonella spp
Staphylococcus aureus
Stenotrophomonas maltophilia
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
Viridans streptococci
Table 1. Most Common Bacterial Pathogens on Basis of Age and Predisposing Risks [1, 13] (Open Table in a new window)
Risk or Predisposing Factor |
Bacterial Pathogen |
Age 0-4 weeks |
Streptococcus agalactiae (GBS) Escherichia coli K1 Listeria monocytogenes |
Age 4-12 weeks |
S agalactiae E coli Haemophilus influenzae Streptococcus pneumoniae Neisseria meningitidis |
Age 3 months to 18 years |
N meningitidis S pneumoniae H influenzae |
Age 18-50 years |
S pneumoniae N meningitidis H influenzae |
Age >50 years |
S pneumoniae N meningitidis L monocytogenes Aerobic gram-negative bacilli |
Immunocompromised state |
S pneumoniae L monocytogenes Pseudomonas aeruginosa Mycobacterium tuberculosis N meningitidis Gram-negative bacteria |
Intracranial manipulation, including neurosurgery |
Staphylococcus aureus Coagulase-negative staphylococci Aerobic gram-negative bacilli, including Pseudomonas aeruginosa |
Basilar skull fracture |
S pneumoniae H influenzae Group A streptococci |
CSF shunts |
Coagulase-negative staphylococci S aureus Aerobic gram-negative bacilli Propionibacterium acnes |
CSF = cerebrospinal fluid; GBS = group B streptococcus. |
|
Some of the more common bacterial pathogens causing meningitis are elaborated below, but any bacteria is capable of causing meningitis
H influenzae meningitis
H influenzae is a small, pleomorphic, gram-negative coccobacillus that is frequently found as part of the normal flora in the upper respiratory tract. The organism can spread from one individual to another in airborne droplets or by direct contact with secretions. Meningitis is the most serious acute manifestation of systemic infection with H influenzae.
In the past, H influenzae was a major cause of meningitis, and the encapsulated type b strain of the organism (Hib) accounted for most cases. Since the introduction of the Hib vaccine in the United States in 1990, H influenzae meningitis is rare in the United States and Western Europe, where use of the vaccine is common. In areas where the vaccine is not widely used, H influenza is a common cause of meningitis, particularly in children aged 2 months to 6 years. [11]
The isolation of H influenzae in adults suggests the presence of an underlying medical disorder, such as the following:
-
Paranasal sinusitis
-
Otitis media
-
Alcoholism
-
CSF leak after head trauma
-
Functional or anatomic asplenia
-
Hypogammaglobulinemia
(See Haemophilus Meningitis.)
Listeria monocytogenes meningitis
Listeria monocytogenes, a small gram-positive bacillus, accounts for 3% of bacterial meningitis cases and is associated with one of the highest mortality rates at 20%. [14] Widely distributed in nature, this pathogen has been found in the stool of 5% of healthy adults, with most infections believed to be food-related. [11]
Known as a common food contaminant, L monocytogenes has a recovery rate of up to 70% from raw meat, vegetables, and various food products. Outbreaks of listeriosis have been linked to the consumption of contaminated items such as coleslaw, milk, cheese, and alfalfa tablets.
Groups at risk include the following:
-
Pregnant individuals
-
Infants and children
-
Elderly individuals (>60 years)
-
Patients with alcoholism
-
Adults who are immunosuppressed (eg, steroid users, transplant recipients, or persons with AIDS)
-
Individuals with chronic liver and renal disease
-
Individuals with diabetes
-
Persons with iron-overload conditions (eg, hemochromatosis or transfusion-induced iron overload)
Meningitis caused by gram-negative bacilli
Aerobic gram-negative bacilli include the following [1, 9] :
-
Escherichia coli
-
Klebsiella pneumoniae
-
Serratia marcescens
-
P aeruginosa
-
Salmonella species
Gram-negative bacilli can cause meningitis in certain groups of patients. E coli is a common agent of meningitis among neonates. Other predisposing risk factors for meningitis associated with gram-negative bacilli include the following:
-
Neurosurgical procedures or intracranial manipulation
-
Old age
-
Immunosuppression
-
>p?High-grade gram-negative bacillary bacteremia
-
Disseminated strongyloidiasis
Disseminated strongyloidiasis has been reported as a classic cause of gram-negative bacillary bacteremia, as a result of the translocation of gut microflora with the Strongyloides stercoralis larvae during hyperinfection syndrome.
Meningococcal meningitis
N meningitidis is a gram-negative diplococcus that is carried in the nasopharynx of otherwise healthy individuals. It initiates invasion by penetrating the airway epithelial surface. [1] The precise mechanism by which this occurs is unclear, but recent viral or mycoplasmal infection has been reported to disrupt the epithelial surface and facilitate invasion by meningococcus.
Most sporadic cases of meningococcal meningitis (95-97%) are caused by serogroups B, C, and Y, whereas the A and C strains are observed in epidemics (< 3% of cases). N meningitidis is one of the leading causes of bacterial meningitis in children and young adults, but the incidence has decreased with use of the conjugate meningococcal vaccine. [1, 15]
Risk factors for meningococcal meningitis include the following:
-
Deficiencies in terminal complement components (eg, membrane attack complex, C5-C9), which increases attack rates but is associated with surprisingly lower mortality rates
-
Properdin defects that increase the risk of invasive disease
-
Antecedent viral infection, chronic medical illness, corticosteroid use, and active or passive smoking
-
Crowded living conditions, as is observed in college dormitories (college freshmen living in dormitories are at highest risk) and military facilities, which has been reported in clustering of cases
(See Meningococcal Meningitis.)
Staphylococcal meningitis
Staphylococci are gram-positive cocci that are part of the normal skin flora. Meningitis caused by staphylococci is associated with the following risk factors [11, 16] :
-
Neurosurgery
-
Head trauma
-
Presence of CSF shunts
-
Infective endocarditis and paraspinal infection
S epidermidis is the most common cause of meningitis in patients with CNS (ie, ventriculoperitoneal) shunts.
Pneumococcal meningitis
S pneumoniae, a gram-positive coccus, is the most common bacterial cause of meningitis in middle-aged adults and the elderly. [1, 11, 17] In addition, it is the most common bacterial agent in meningitis associated with basilar skull fracture and CSF leak. It may be associated with other focal infections, such as pneumonia, sinusitis, or endocarditis (as, for example, in Austrian syndrome, which is the triad of pneumococcal meningitis, endocarditis, and pneumonia).
S pneumoniae is a common colonizer of the human nasopharynx; it is present in 5-10% of healthy adults and 20-40% of healthy children. It causes meningitis by escaping local host defenses and phagocytic mechanisms, either through choroid plexus seeding from bacteremia or through direct extension from sinusitis or otitis media.
Patients with the following conditions are at increased risk for S pneumoniae meningitis:
-
Hyposplenism
-
Hypogammaglobulinemia
-
Multiple myeloma
-
Glucocorticoid treatment
-
Defective complement (C1-C4)
-
Diabetes mellitus
-
Renal insufficiency
-
Alcoholism
-
Malnutrition
-
Chronic liver disease
Streptococcus agalactiae meningitis
Streptococcus agalactiae (group B streptococcus [GBS]) is a gram-positive coccus that inhabits the lower GI tract. It also colonizes the female genital tract at a rate of 5-40%, which explains why it is the most common agent of neonatal meningitis (associated with 70% of cases). Routine testing and treatment of pregnant females for GBS has led to a decrease in neonatal meningitis with this organism. [11]
Predisposing risks in adults include the following:
-
Diabetes mellitus
-
Pregnancy
-
Alcoholism
-
Hepatic failure
-
Renal failure
-
Corticosteroid treatment
In 43% of adult cases, however, no underlying disease is present.
Viral meningitis
Viral meningitis typically is less severe than acute bacterial meningitis and may present with fever and myalgias before progressing to typical meningitis symptoms including headache and nuchal rigidity. Delirium, confusion, and neurological deficits usually are absent due to the preservation of brain tissue. [18]
Various viruses, such as adenovirus, astrovirus, and enteroviruses, can cause meningitis, with different modes of transmission and seasonal patterns. [19, 20] Herpesviruses, including Epstein-Barr virus and cytomegalovirus, as well as HIV, can also lead to meningitis in certain populations. Arthropod-borne viruses like West Nile virus and St Louis encephalitis virus can cause aseptic meningitis syndrome. Other pathogens such as Lymphocytic choriomeningitis virus and mumps virus may also be responsible for aseptic meningitis. Travelers returning from Mediterranean countries during the summer should be aware of Toscana virus meningitis or encephalitis. Diagnosing these viral infections may involve performing paired serologies and CSF PCR. [21, 22, 23, 24, 25, 26]
Please see Viral Meningitis and Aseptic Meningitis.)
Aseptic Meningitis
Aseptic meningitis, although sometimes used interchangeably with viral meningitis, generally describes acute meningitis resulting from pathogens other than the typical bacteria responsible for acute bacterial meningitis; it is one of the most common infections of the meninges. Although viruses are the most common cause of aseptic meningitis, it also can be caused by bacteria, fungi, and parasites. [1] Partially treated bacterial meningitis accounts for a large number of meningitis cases with a negative microbiologic workup.
In many cases, a cause of meningitis is not apparent after initial evaluation, and the disease therefore is classified as aseptic meningitis. These patients characteristically have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
When the cause of aseptic meningitis is discovered, the disease can be reclassified according to its etiology. If appropriate diagnostic methods are performed, a specific viral etiology is identified in 55-70% of cases of aseptic meningitis. However, the condition also can be caused by bacterial, fungal, mycobacterial, and parasitic agents.
If, after an extensive workup, aseptic meningitis is found to have a viral etiology, it can be reclassified as a form of acute viral meningitis (eg, enteroviral meningitis or herpes simplex virus [HSV] meningitis). [27]
(See Aseptic Meningitis.)
Table 2. Infectious Agents Causing Aseptic Meningitis [1, 28] (Open Table in a new window)
Category |
Agent |
||
Bacteria |
Partially treated bacterial meningitis Borrelia burgdorferi Brucella spp Ehrlichia spp Leptospira spp Listeria monocytogenes Mycobacterium tuberculosis Mycoplasma pneumoniae Nocardia spp Rickettsia rickettsii Treponema pallidum |
||
Parasites |
Acanthamoeba spp Angiostrongylus cantonensis Balamuthia spp Baylisascaris procyonis Gnathostoma spinigerum Naegleria fowleri Strongyloides stercoralis Taenia solium (cysticercosis) Toxocara spp |
||
Fungi |
Aspergillus spp Blastomyces dermatitidis Candida spp Cryptococcus neoformans Coccidioides immitis Histoplasma capsulatum |
||
Viruses |
Enterovirus |
Coxsackievirus A Coxsackievirus B Echovirus Enterovirus 68-71 Poliovirus |
|
Herpesvirus (HSV) |
Cytomegalovirus Epstein-Barr virus HHV-6 and HHV-7 HSV-1 and HSV-2 Varicella-zoster virus |
||
Paramyxovirus |
Measles virus Mumps virus |
||
Togavirus |
Rubella virus |
||
Flavivirus |
Japanese encephalitis virus St Louis encephalitis virus West Nile virus |
||
Bunyavirus |
California encephalitis virus La Crosse encephalitis virus |
||
Alphavirus |
Eastern equine encephalitis virus Venezuelan encephalitis virus Western equine encephalitis virus |
||
Reovirus |
Colorado tick fever virus |
||
Arenavirus |
LCM virus |
||
Rhabdovirus |
Rabies virus |
||
Retrovirus |
HIV-1 HIV-2 |
||
HHV = human herpesvirus; HSV = herpes simplex virus; LCM = lymphocytic choriomeningitis. |
|||
See Meningitis in HIV.
Subacute and Chronic Meningitis
Chronic meningitis is a constellation of signs and symptoms of meningeal irritation associated with CSF pleocytosis that persists for longer than 4 weeks. [8]
Chronic meningitis can be caused by a wide range of infectious and noninfectious etiologies (see Table 3 below). [8]
Table 3. Causes of Chronic Meningitis [8] (Open Table in a new window)
Category |
Agent |
Bacteria |
Actinomyces spp Borrelia burgdorferi Brucella spp Francisella tularensis Mycobacterium tuberculosis Nocardia spp Treponema pallidum |
Fungi |
Aspergillus spp Blastomyces dermatitidis Candida albicans Cryptococcus neoformans Coccidioides immitis Histoplasma capsulatum Sporothrix schenckii |
Parasites |
Acanthamoeba spp Angiostrongylus cantonensis Baylisascarisprocyonis Echinococcus granulosus Gnathostoma spinigerum Naegleria fowleri Schistosoma spp Strongyloides stercoralis |
Acanthamoeba and Balamuthia cause granulomatous amebic encephalitis, which is a subacute opportunistic infection that spreads hematogenously from the primary site of infection (skin or lungs) to the CNS and causes an encephalitis syndrome. These cases can be difficult to distinguish from culture-negative meningitis. [8]
Angiostrongylus cantonensis, the rat lungworm, can cause eosinophilic meningitis (pleocytosis with more than 10% eosinophils) in humans. The adult parasite resides in the lungs of rats. Its eggs hatch, and the larval stages are expelled in the feces. The larvae develop in the intermediate host, usually land snails, freshwater prawns, and crabs. Humans acquire the infection by ingesting raw mollusks. [8]
Baylisascaris procyonis is an ascarid parasite that is prevalent in the raccoon populations in the United States and rarely causes human eosinophilic meningoencephalitis. Human infections occur after accidental ingestion of food products contaminated with raccoon feces. [8]
Blastomyces dermatitidis is a dimorphic fungus that has been reported to be endemic in North America (eg, in the Mississippi and Ohio River basins). It also has been isolated from parts of Central America, South America, the Middle East, and India. Its natural habitat is not well defined. Soil that is rich in decaying matter and environments around riverbanks and waterways have been demonstrated to harbor B dermatitidis during outbreaks and are thought to be risk factors for acquiring the infection. [8]
Inhalation of the conidia establishes a pulmonary infection. Dissemination may occur in certain individuals, including those with underlying immune deficiency (eg, from HIV or pharmaceutical agents) and extremes of age, and may involve the skin, bones and joints, genitourinary tract, and CNS. Involvement of the CNS occurs in fewer than 5% of cases.
Borrelia burgdorferi, a tick-borne spirochete, is the agent of Lyme disease, the most common vector-borne disease in the United States. Meningitis may be part of a triad of neurologic manifestations of Lyme disease that also includes cranial neuritis and radiculoneuritis. Lyme disease meningitis typically is associated with a facial palsy that can be bilateral. As many as 8% of children and some adults with Lyme disease develop meningitis. [1]
Brucellae are small gram-negative coccobacilli that cause zoonoses as a result of infection with Brucella abortus, Brucella melitensis, Brucella suis, or Brucella canis. Transmission to humans occurs after direct or indirect exposure to infected animals (eg, sheep, goats, or cattle). Direct infection of the CNS occurs in fewer than 5% of cases, with most patients presenting with acute or chronic meningitis. [8]
Persons at risk for brucellosis include individuals who had contact with infected animals or their products (eg, through intake of unpasteurized milk products). Veterinarians, abattoir workers, and laboratory workers dealing with these animals also are at risk. [8]
Candida species are ubiquitous in nature. They are normal commensals in humans and are found in the skin, the GI tract, and the female genital tract. The most common species is Candida albicans, but the incidence of non-albicans candidal infections (eg, Candida tropicalis) is increasing, including species with antifungal resistance (eg, Candida krusei and Candida glabrata). [8]
Involvement of the CNS usually follows hematogenous dissemination. The most important predisposing risks for acquiring disseminated candidal infection appear to be iatrogenic (eg, the administration of broad-spectrum antibiotics and the use of indwelling devices such as urinary and vascular catheters). Prematurity in neonates is considered a predisposing risk factor as well. Infection also may follow neurosurgical procedures, such as placement of ventricular shunts. [16, 8]
Coccidioides immitis is a soil-based, dimorphic fungus that exists in mycelial and yeast (spherule) forms. Persons at risk for coccidioidal meningitis include individuals exposed to the endemic regions (eg, tourists and local populations) and those with immune deficiency (eg, persons with AIDS and organ transplant recipients). [8]
Cryptococcus neoformans is an encapsulated, yeastlike fungus that is ubiquitous. It has been found in high concentrations in aged pigeon droppings and pigeon nesting places. The 4 serotypes are designated A through D, with the A serotype causing most human infections. Onset of cryptococcal meningitis may be acute, especially among patients with AIDS. [8]
Numerous cases occur in healthy hosts (eg, persons with no known T-cell defect) [8] ; however, approximately 50-80% of cases occur in immunocompromised hosts. At particular risk are individuals with defects of T-cell–mediated immunity, such as persons with AIDS, organ transplant recipients, and other patients who use steroids, cyclosporine, and other immunosuppressants. Cryptococcal meningitis also has been reported in patients with idiopathic CD-4 lymphopenia, Hodgkin disease, sarcoidosis, and cirrhosis.
Gnathostoma spinigerum, a GI parasite of wild and domestic dogs and cats, may cause eosinophilic meningoencephalitis. Humans acquire the infection after ingesting undercooked infected fish and poultry. [8]
Histoplasma capsulatum is one of the dimorphic fungi that exist in mycelial and yeast forms. It usually is found in soil and occasionally can cause a chronic meningitis. The preferred means of making the diagnosis is CSF histoplasma antigen detection. [8]
M tuberculosis is an acid-fast bacillus that causes a broad range of clinical illnesses that can affect virtually any organ of the body. It is spread through airborne droplet nuclei, and it infects one third of the world’s population. Involvement of the CNS with tuberculous meningitis usually is caused by rupture of a tubercle into the subarachnoid space.
Tuberculous meningitis always should be considered in the differential diagnosis of patients with aseptic meningitis or chronic meningitis syndromes, especially those with basilar meningitis, symptoms of more than 5 days’ duration, or cranial nerve palsies. If tuberculous meningitis is suspected, antituberculosis therapy, with or without steroids, should be empirically started. [8] (See Tuberculous Meningitis.) [29]
Sporothrix schenckii is an endemic dimorphic fungus that often is isolated from soil, plants, and plant products. Human infections are characteristically lymphocutaneous. Extracutaneous manifestations of sporotrichosis may occur, though meningeal sporotrichosis, which is the most severe form, is a rare complication. AIDS is a reported underlying risk factor in many described cases and is associated with a poor outcome. [8]
Treponema pallidum is a slender, tightly coiled spirochete that is usually acquired by sexual contact. Other modes of transmission include direct contact with an active lesion, passage through the placenta, and blood transfusion (rare). [8]
Infection with free-living amoebas is an infrequent but often life-threatening human illness, even in immunocompetent individuals. N fowleri is the only species of Naegleria recognized to be pathogenic in humans, and it is the agent of primary amebic meningoencephalitis (PAM). The parasite has been isolated in lakes, pools, ponds, rivers, tap water, and soil. [8]
Infection occurs when a person is swimming or playing in contaminated water sources (eg, inadequately chlorinated water and sources associated with poor decontamination techniques). The N fowleri amebas invade the CNS through the nasal mucosa and cribriform plate. [30]
PAM occurs in 2 forms. The first is characterized by an acute onset of high fever, photophobia, headache, and altered mental status, similar to bacterial meningitis, occurring within 1 week after exposure. Because it is acquired via the nasal area, olfactory nerve involvement may manifest as abnormal smell sensation. Death occurs in 3 days in patients who are not treated. The second form, the subacute or chronic form, consists of an insidious onset of low-grade fever, headache, and focal neurologic signs. Duration of illness is weeks to a few months. [30]
Noninfectious Meningitis
Noninfectious meningitis can be attributed to various factors such as noninfectious disorders, drugs, or vaccines, resulting in subacute or chronic presentations. The symptoms of noninfectious meningitis, which include headache, fever, and nuchal rigidity, are similar to those observed in other forms of meningitis. Although the severity and duration of symptoms may vary, noninfectious meningitis typically is less severe compared to acute bacterial meningitis. [31]
Recurrent Meningitis
Recurrent meningitis usually is caused by bacteria, viruses, or noninfectious conditions. [9]
Recurrent viral meningitis
Recurrent viral meningitis most often is caused by Herpes simplex virus type 2 (HSV-2; also known as Mollaret meningitis)
In cases where HSV-2 is the identified cause, patients may experience recurrent episodes marked by symptoms like fever, nuchal rigidity, and lymphocytic pleocytosis in cerebrospinal fluid (CSF). Each bout typically lasts for 2 to 5 days before spontaneously resolving. Patients also exhibit additional neurological deficits, including altered sensorium, seizures, and cranial nerve palsies, suggesting a diagnosis of meningoencephalitis.
Whenever feasible, addressing the root cause is a key aspect of treatment. Acyclovir is the recommended treatment for Mollaret meningitis, with most patients achieving complete recovery.
Recurrent acute bacterial meningitis
Acute bacterial meningitis may recur if it arises from an unresolved congenital or acquired defect in the skull base or spine. If the defect is a result of an injury, meningitis may manifest years later.
Rarely, recurrent bacterial meningitis (usually due to Streptococcus pneumoniae or Neisseria meningitidis) results from a deficiency in the complement system. Treatment is the same as that used in patients without complement deficits. Vaccination against S. pneumoniae and N. meningitidis (given according to Centers for Disease Control and Prevention [CDC] recommendations for patients with complement deficits) may reduce likelihood of infection.
Recurrent bacterial meningitis is treated with antibiotics and dexamethasone.
Other recurrent meningitides
Acute meningitis secondary to nonsteroidal anti-inflammatory drugs (NSAIDs) or other drugs may recur when the causative drug is used again.
Meningitis caused by rupture of a brain cyst may also recur.
Additional Causes of Meningitis
Congenital malformation of the stapedial footplate has been implicated in the development of meningitis. Head and neck surgery, penetrating head injury, comminuted skull fracture, and osteomyelitic erosion infrequently may result in direct implantation of bacteria into the meninges. Skull fractures can tear the dura and cause a CSF fistula, especially in the region of the frontal ethmoid sinuses. Patients with any of these conditions are at risk for bacterial meningitis. [1]
Epidemiology
Epidemiology of Bacterial Meningitis
Bacterial meningitis affects around 4,100 individuals annually in the United States causes 500 deaths, equating to an overall annual incidence rate of 1.33 cases per 100,000 people. [32] The incidence of meningitis varies globally, with developing nations experiencing rates up to 10 times higher than developed countries due to limited access to preventive measures. In the United States, there is a higher reported incidence of meningitis among Black individuals compared with White and Hispanic populations. [33]
During 1998 to 2007, there was a 31% decrease in meningitis incidence in the United States, attributed to vaccination programs. The introduction of the Hib conjugate vaccine for infants in the early 1990s led to a 55% reduction in bacterial meningitis rates, with reported cases of invasive H influenzae disease in children under 5 dropping significantly. Whereas these reductions have been significant in developed nations, the impact has been less profound in developing countries where Hib vaccination is not as widespread. [14] The implementation of pneumococcal vaccines and universal screening for group B streptococcus in pregnant women has further lowered the incidence of meningitis among young children; however, the burden of bacterial meningitis has shifted to impact older adults.
Since 1988, the incidence of H influenzae meningitis in the Netherlands has decreased significantly due to the implementation of Hib vaccination. The incidence of N meningitidis meningitis also has decreased, mainly due to the MenC vaccination. However, S pneumoniae has become the most common pathogen causing bacterial meningitis, with interventions being less effective compared to those for H influenzae. The use of conjugate vaccines has led to a decline in meningitis cases in non-vaccinated populations. Overall, although there have been significant reductions in meningitis cases in preschool and school-aged children, rates remain high in infants, older adults, and the elderly. [34]
Table 4. Changing Epidemiology of Acute Bacterial Meningitis in United States* [14] (Open Table in a new window)
Bacteria |
1978-1981 |
1986 |
1995 |
1998-2007 |
|
Haemophilus influenzae |
48% |
45% |
7% |
6.7% |
|
Listeria monocytogenes |
2% |
3% |
8% |
3.4% |
|
Neisseria meningitidis |
20% |
14% |
25% |
13.9% |
|
Streptococcus agalactiae (group B streptococcus) |
3% |
6% |
12% |
18.1% |
|
Streptococcus pneumoniae |
13% |
18% |
47% |
58% |
|
*Nosocomial meningitis is not included; these data include only the 5 major meningeal pathogens. |
|||||
Table 5. Changing Epidemiology of Bacterial Meningitis Since Introduction of Conjugate Vaccines in The Netherlands [34] (Open Table in a new window)
Bacteria |
1989–1993* |
2014–2019* |
Haemophilus influenzae |
1.57 (Hib 96.4% of H influenzae meningitis in 1993) |
0.14 |
Hib |
1.44 in 1993 |
0.04 in 2001-2002 |
Listeria monocytogenes |
0.10 |
0.05 |
Neisseria meningitidis |
2.87 |
0.20 |
Streptococcus agalactiae (group B streptococcus) |
34.84 |
42.48 |
Streptococcus pneumoniae |
1.10 |
1.48 |
*Per 100,000 episodes |
(See Meningococcal Meningitis.)
Epidemiology of Aseptic and Viral Meningitis
Viruses are the major cause of aseptic meningitis. In the United States, enteroviral meningitis affects about 75,000 individuals annually, representing more than half of all cases of meningitis. [32]
Aseptic meningitis has a reported incidence of 10.9 cases per 100,000 person-years. It occurs in individuals of all ages but is more common in children, especially during summer. No racial differences are reported. [35]
Viral meningitis was the most prevalent form of meningitis in patients aged 16 years and older, followed by bacterial cases and those with unknown causes in a multicenter prospective observational study in England. The research emphasized the significance of early lumbar puncture in determining the specific cause and reducing hospital stays. Patients diagnosed with viral meningitis experienced a considerable loss in quality-adjusted life-years. [36]
Nonpolio Enteroviruses (NPEVs) include the coxsackieviruses, echoviruses, and newer numbered EVs (a total of 67 distinct serotypes). In the United States alone, the NPEVs cause an estimated 10 to 15 million symptomatic infections annually. [35] [37]
(See Aseptic Meningitis and Viral Meningitis.)
Prognosis
Patients with bacterial meningitis who present with an impaired level of consciousness, hypotension, or seizures are at increased risk for neurologic sequelae or death. [19]
In bacterial meningitis, several risk factors are associated with death and with neurologic disability. [1] A risk score has been developed and validated in adults with bacterial meningitis. This score includes the following variables, which are associated with an adverse clinical outcome:
-
Older age
-
Increased heart rate
-
Lower Glasgow Coma Scale score
-
Cranial nerve palsies
-
CSF leukocyte count lower than 1000/μL
-
Gram-positive cocci on CSF Gram stain
Bacterial meningitis can result in severe neurologic complications in up to 30% of survivors, [12] underscoring the crucial importance of vigilant monitoring to detect and address these issues promptly. Mortality rates vary across age groups, with the highest fatalities seen in infants, decreasing in midlife, and rising again in older age groups, leaving 1 in 10 cases fatal and 1 in 7 survivors facing significant disabilities like deafness or brain injury.
A comprehensive review spanning nearly a century and multiple countries highlighted key pathogens like Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae in causing meningitis episodes. The overall case fatality ratio has shown a progressive decline, with Listeria monocytogenes and pneumococci associated with higher mortality rates. [38] Notably, S pneumoniae meningitis has a fatality rate of 19-26%, [39] and Haemophilus influenzae type b (Hib) cases in children range from 3-6% fatality. Older individuals face elevated risks. [40]
(See Haemophilus Meningitis.)
Increases in meningococcal disease cases, particularly due to serogroup Y infections, have prompted public health alerts to raise awareness and advocate for preventive measures. Timely vaccination and medical attention are vital in managing these serious bacterial infections, which can lead to long-term complications such as brain damage and coma, with up to 30% of survivors experiencing neurological sequelae. Ensuring vigilant monitoring and immediate intervention remains paramount in mitigating the impacts of bacterial meningitis. [41] (See Meningococcal Meningitis.)
Serious complications include the following:
-
Hearing loss
-
Cortical blindness
-
Other cranial nerve dysfunction
-
Paralysis
-
Muscular hypertonia
-
Ataxia
-
Multiple seizures
-
Mental motor impairment
-
Focal paralysis
-
Ataxia
-
Subdural effusions
-
Hydrocephalus
-
Cerebral atrophy
Risk factors for hearing loss after pneumococcal meningitis are female sex, older age, severe meningitis, and infection with certain pneumococcal serotypes (eg, 12F). [42] Delayed complications include the following:
-
Decreased hearing or deafness
-
Delayed cerebral thrombosis [43]
-
Other cranial nerve dysfunctions
-
Multiple seizures
-
Focal paralysis
-
Subdural effusions
-
Hydrocephalus
-
Intellectual deficits
-
Ataxia
-
Blindness
-
Waterhouse-Friderichsen syndrome
-
Peripheral gangrene
Seizures are a common and significant complication of meningitis, occurring in about one fifth of patients, with a higher incidence (40%) in those younger than 1 year. Half of patients experiencing seizures may have recurrent episodes, leading to adverse outcomes such as diffuse CNS ischemic injury or systemic complications.
The prognosis for patients with meningitis caused by opportunistic pathogens heavily depends on the host's immune function, requiring many survivors to undergo lifelong suppressive therapy post-recovery. In viral meningitis cases without encephalitis, the mortality rate is less than 1%. However, individuals with deficient humoral immunity, like agammaglobulinemia, facing enteroviral meningitis may encounter fatal outcomes. Fortunately, patients with viral meningitis typically have a favorable recovery prognosis, with poorer outcomes seen in those at the extremes of age (under 2 or over 60 years) and individuals with significant comorbidities or underlying immunodeficiency.
Patient Education
Patients and parents of young children should be educated about the benefits of vaccination in preventing meningitis. Vaccination against N meningitidis is recommended for all US college students.
Close contacts of patients with known or suspected N meningitidis or Hib meningitis may require education regarding the need for prophylaxis. All contacts should be instructed to come to the emergency department immediately at the first sign of fever, sore throat, rash, or symptoms of meningitis. Rifampin prophylaxis only eradicates the organism from the nasopharynx; it is ineffective against invasive disease.
-
Pneumococcal meningitis in a patient with alcoholism. Courtesy of the CDC/Dr. Edwin P. Ewing, Jr.
-
Acute bacterial meningitis. This axial nonenhanced computed tomography scan shows mild ventriculomegaly and sulcal effacement.
-
Acute bacterial meningitis. This axial T2-weighted magnetic resonance image shows only mild ventriculomegaly.
-
Acute bacterial meningitis. This contrast-enhanced, axial T1-weighted magnetic resonance image shows leptomeningeal enhancement (arrows).
-
Chronic mastoiditis and epidural empyema in a patient with bacterial meningitis. This axial computed tomography scan shows sclerosis of the temporal bone (chronic mastoiditis), an adjacent epidural empyema with marked dural enhancement (arrow), and the absence of left mastoid air.
-
Subdural empyema and arterial infarct in a patient with bacterial meningitis. This contrast-enhanced axial computed tomography scan shows left-sided parenchymal hypoattenuation in the middle cerebral artery territory, with marked herniation and a prominent subdural empyema.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Treatment of Subacute Meningitis
- Treatment of Bacterial Meningitis
- Treatment of Viral Meningitis
- Treatment of Fungal Meningitis
- Treatment of Tuberculous Meningitis
- Treatment of Syphilitic Meningitis
- Treatment of Parasitic Meningitis
- Treatment of Lyme Meningitis
- Prevention
- Consultations
- Long-Term Monitoring
- Activity
- Treatment of Noninfectious Meningitis
- Treatment of Recurrent Meningitis
- Treatment of Other Causes of Recurrent Meningitis
- Show All
- Guidelines
- Medication
- Medication Summary
- Sulfonamides
- Tetracyclines
- Carbapenems
- Fluoroquinolones
- Antibiotics, Miscellaneous
- Glycopeptides
- Aminoglycosides
- Penicillins, Amino
- Penicillins, Natural
- Cephalosporins, 3rd Generation
- Antivirals, CMV
- Antivirals, Other
- Antifungals, Systemic
- Antituberculous Agents
- Vaccines, Inactivated, Bacterial
- Corticosteroids
- Diuretics, Osmotic Agents
- Diuretics, Loop
- Anticonvulsants, Hydantoins
- Anticonvulsants, Barbiturates
- Anticonvulsants, Other
- Show All
- Media Gallery
- Tables
- References