Overview
Progressive multifocal leukoencephalopathy (PML) is a severe viral infection of the central nervous system, primarily affecting individuals with severe immunosuppression due to conditions such as advanced HIV infection, cancer treatments, autoimmune diseases, and organ transplants. [1, 2] Caused by the John Cunningham virus (JCV), which was identified in 1967, JCV targets brain oligodendrocytes, leading to demyelination. JCV occurs almost exclusively in immunosuppressed individuals, including those with AIDS, hematologic malignancies, autoimmune diseases, or those undergoing organ transplantation. PML has also been reported in patients receiving immune therapy with monoclonal antibodies and various other immunosuppressants. [3]
PML is associated with both HIV-1 and HIV-2, [4, 5] accounting for almost 85% of the total cases in HIV-infected patients, with a prevalence of around 4–5%. [6, 7, 8] It is considered one of the AIDS-defining illnesses in HIV-infected patients. The introduction of highly active antiretroviral therapy (HAART) has significantly reduced the incidence of PML in patients with HIV by improving immune function, although it can also trigger PML-IRIS (immune reconstitution inflammatory syndrome), an autoimmune response as the immune system recovers. HIV-associated PML can also occur during immune recovery following the initiation of HAART, associated with an inflammatory reaction in brain lesions and contrast enhancement on neuroimaging studies. [9, 10, 11, 12, 13] Most patients with HIV infection develop PML when their CD4 cell count is low (< 200/µL), although there are few reports of PML in patients with better immunological function (CD4 counts > 500/µL). [14, 15] Recurrences of PML have been reported even after many years of immune recovery in HIV. [16]
The JC Virus (JCV) is a common virus that establishes a persistent, asymptomatic infection in many adults, with its prevalence increasing with age. [17] It is likely transmitted via the fecal-oral route during childhood, leading to latent infections in various body sites, including the kidneys and possibly the brain. Although JCV is generally harmless in healthy individuals, it can become pathogenic in those with compromised immune systems, undergoing genetic changes that enable it to attack the nervous system.
PML is rare and occurs in people undergoing chronic corticosteroid or immunosuppressive therapy for organ transplant, or individuals with cancer (such as Hodgkin's disease or lymphoma). Individuals with autoimmune conditions such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus—some of whom are treated with biological therapies that allow JC virus reactivation—are at risk for PML as well.
The primary reservoirs of disease-associated JCV are not well understood, but the excretion of the virus in urine, which occurs in about 30% of healthy individuals, does not correlate with an increased risk of developing PML. The bone marrow has been suggested as a potential reservoir, especially in cases of natalizumab-associated PML, where the virus might undergo necessary genetic changes.
JCV may also spread to the brain via extracellular vesicles (EVs), which allow the virus to enter cells independently of viral receptors and evade the immune system. Although JCV has been found associated with EVs in vitro and in the plasma of HIV-infected individuals, the role of EVs in JCV transmission and the pathogenesis of PML remains unclear.
The JCV genome encodes several proteins that facilitate its replication and modify its infectivity. Genetic rearrangements in the noncoding control region (NCCR) of the virus are crucial for the development of PML, enhancing the virus's ability to replicate. Mutations in the VP1 capsid protein can alter the virus's cell-binding capabilities, potentially aiding its entry into brain cells.
Host genetics also influence susceptibility to PML, with certain genetic variants, including those in the human leukocyte antigen (HLA) system and other immune-related genes, potentially increasing the risk of developing the disease.
Pathophysiology
JC Virus (JCV) commonly establishes a persistent, asymptomatic infection in a significant portion of the adult population, with its prevalence increasing with age. [17] Typically transmitted via the fecal-oral route during childhood, JCV remains latent in various body sites such as the kidneys and potentially the brain. Although generally harmless in healthy individuals, JCV can become pathogenic in those with compromised immune systems, such as those with HIV/AIDS, through genetic changes that enable it to attack the nervous system.
The primary reservoirs for disease-associated JCV are not fully understood. [17] About 30% of healthy individuals excrete the virus in their urine, but this does not correlate with an increased risk of developing progressive multifocal leukoencephalopathy (PML), a severe neurologic condition caused by JCV. The bone marrow has been suggested as a potential reservoir, particularly in cases of natalizumab-associated PML, where the virus may undergo necessary genetic alterations.
JCV may also spread to the brain via extracellular vesicles (EVs), which allow the virus to enter cells without using viral receptors, thereby evading the immune system. [17] Despite evidence of JCV association with EVs in vitro and in the plasma of HIV-infected individuals, the exact role of EVs in the transmission of JCV and the pathogenesis of PML remains unclear.
The JCV genome encodes several proteins essential for its replication and infectivity. Genetic rearrangements in the noncoding control region (NCCR) of the virus are critical for PML development, enhancing the virus's replication capabilities. Additionally, mutations in the VP1 capsid protein may modify the virus's ability to bind to cells, potentially facilitating its entry into brain cells.
Host genetics also influence susceptibility to PML, with certain genetic variants, particularly those in the human leukocyte antigen (HLA) system and other immune-related genes, increasing the risk of developing the disease. [17]
Progressive multifocal leukoencephalopathy (PML) is triggered by the reactivation of the endemic JC polyomavirus. Although up to 90% of healthy individuals have serum antibodies to this virus, less than 10% show evidence of ongoing viral replication. [18] The virus is thought to enter the body via the respiratory or oral route, becoming latent in the kidneys, lymphoreticular tissues, and brain. The primary infection is asymptomatic, but periods of viral replication can occur without symptoms and be detected when shed in the urine. Reactivation in the setting of immune suppression leads to viral replication and dissemination to the brain, or reactivation may occur directly in the brain.
A study comparing HIV-negative controls and HIV-positive patients with or without PML found that a third of individuals from all subgroups had JC virus DNA in their urine. [19] In HIV-positive patients with PML, JC virus DNA was detected in 43% of lymphocyte samples and 63% of plasma samples. In contrast, in HIV-positive patients without PML, JC virus DNA was detected in only 13% of lymphocyte samples and 22% of plasma samples. No JC viral DNA was found in lymphocyte or plasma samples of HIV-negative controls.
HIV gene products, such as Tat, may transactivate the JC viral promoter directly, providing a pathogenic mechanism beyond general immunosuppression. The viral infection in oligodendrocytes is lytic, involving DNA replication and synthesis of viral capsid proteins within the cell. The virus infects other cells from a central nidus in a circumferential manner, leading to the expansion of the demyelinating lesion. Infected astrocytes enlarge and take on a bizarre appearance, resembling tumor cells in giant cell astrocytomas.
In patients with PML who develop immune reconstitution, the entry of JCV-specific T cells, B cells, and monocytes into the CNS at the site of JCV infection helps eliminate the virus. However, a subgroup of patients with PML shows no or limited response despite immune reconstitution. A study of four patients with multiple sclerosis who developed natalizumab-associated PML and granule cell neuronopathy demonstrated that mutations in the VP1 protein of the JCV may generate altered peptides that escape efficient CD4 T-cell recognition, resulting in impaired support for JCV-specific CD8 T cells crucial for eliminating JCV infection of the CNS. [20]
Epidemiology
In a comprehensive study conducted in France from 2010 to 2017, researchers analyzed a cohort of 584 patients with progressive multifocal leukoencephalopathy (PML), marking the largest group studied to that date. [21] The study found a consistent overall incidence rate of PML at 0.11 per 100,000 person-years. The primary risk factors for PML included HIV infection (43.7%), hematological malignancies (21.9%), and chronic inflammatory diseases (20.2%), with less frequent causes being solid organ transplantation, solid tumors, and primary immune deficiencies.
A United States study of 9,675 cases of progressive multifocal leukoencephalopathy (PML) from 1998 to 2005 found that 82% of cases were associated with HIV infection. [22]
The incidence of PML is low in India and Africa, possibly due to diagnostic challenges and differences in JC virus isolates. [23, 24]
Before the advent of highly active antiretroviral therapy (HAART), PML occurred in as many as 4% of AIDS cases. Like other HIV/AIDS-related opportunistic tumors and infections, the incidence of PML has decreased in the HAART era. [25, 26]
According to the Italian NeuroAIDS study 2000-2002 (IRINA), PML is the third most common cause of encephalopathy in HIV-infected patients, after Toxoplasma encephalitis and HIV encephalopathy. [27]
In the United States, the mortality associated with PML has decreased significantly since 1996, when HAART became the standard of care. The PML-associated death rate decreased from 2.76 deaths per 1 million population in 1992-1995 to 0.66 in 2002-2005, in large part because of fewer deaths among HIV-infected patients. [28]
Prognosis
In the pre-HAART era, the prognosis in patients with progressive multifocal leukoencephalopathy (PML) was dismal, with death occurring in approximately 95% of patients within 4–6 months after diagnosis in most cases. Approximately 8% of patients experienced spontaneous recovery.
With the widespread adoption of highly active antiretroviral therapy (HAART), the incidence of PML decreased substantially. In addition, several case series have shown prolonged survival for patients receiving HAART. [29] In a series of 118 consecutive patients from Spain, 63.6% survived for a median of 114 weeks (2.2 y) after diagnosis of PML. [30]
CD4+ T-cell counts less than 100/μL at baseline are associated with a higher mortality rate. Death may result not from the neurologic condition but from end-stage immune deficiency.
In a clinical outcome study of 60 patients with PML (73% HIV+) who were prospectively evaluated for the presence of JC virus (JCV)-specific CD8+ cytotoxic T-lymphocytes (CTL) in blood, estimated probability of survival at 1 year was 52% for HIV+/PML and 58% for HIV-negative patients with PML. The presence of JCV-specific CTLs was associated with a trend toward longer survival in patients with PML. [31]
A study by Lima et al of 24 PML patients whose survival exceeded 5 years found that by the end of the follow-up period, 33% of patients had no significant disability despite persistent symptoms; 25% had slight disability and were living independently; 21% were moderately disabled, requiring some help during activities of daily living; and 21% had moderately severe disability, requiring constant help or institutionalization. [32]
Clinical Presentation
Progressive multifocal leukoencephalopathy (PML) often begins subtly with symptoms such as clumsiness, which may initially appear as the first sign of the disease. [1] Hemiparesis is the most frequently observed symptom, accompanied by other common manifestations including aphasia, dysarthria, and hemianopia. Cognitive impairment, resulting from multifocal cortical damage, affects two thirds of patients, and deficits in sensory, cerebellar, and brain stem functions may also be present.
In patients, particularly those with end-stage HIV infection, headaches and convulsive seizures are rare but can occur. The disease progresses gradually but relentlessly, typically leading to death within 1 to 9 months from the onset of symptoms. The progression of PML involves a steady worsening of focal neurological symptoms, which include behavioral changes, speech difficulties, cognitive decline, motor impairments such as head tremor, [33] and visual disturbances.
Neuropathological assessments reveal the multifocal nature of PML, although clinically, symptoms often appear unifocal initially. MRI scans, however, may show multifocal lesions. Unlike other major opportunistic disorders that produce focal brain lesions, such as cerebral toxoplasmosis or primary CNS lymphoma, which typically worsen over hours or days, PML develops more gradually over several weeks. [34] Nonetheless, PML progresses more rapidly than AIDS dementia complex (ADC). Brainstem involvement is more commonly observed in PML cases associated with AIDS than with other conditions. As the disease progresses, lesions may expand either concentrically or along white matter tracts, leading to worsening symptoms and broader involvement, such as an initial weakness in one leg progressing to hemiparesis. [34] Patients with relatively preserved immune systems may experience a slower progression of the disease compared to those who are more severely immunocompromised.
Although seizures have been considered a rare manifestation of PML, Lima et al found that seizures occurred in 18% of PML patients. [35] Many of the PML patients presenting with seizures had demyelinating lesions immediately adjacent to the cortex. Seizures usually responded well to treatment and did not affect survival.
In PML related to immune reconstitution, onset may occur weeks to months after the initiation of antiretroviral therapy.
Visual symptoms in PML result from visual pathway involvement, not from optic neuritis as in other inflammatory demyelinating diseases. [36]
Physical Examination
Focal neurologic signs include aphasia, hemiparesis, ataxia, cortical blindness, limb apraxia, brainstem symptoms and, less frequently, head tremor. Focal signs tend to be related to posterior brain (eg, occipital lobes). Gait abnormalities occur in up to 65% patients, and cognitive dysfunction is seen at the time of presentation in up to 30% people.
Conjugate gaze abnormalities are common. This is the initial presentation in more than 30% of patients. Abnormalities may progress to quadriparesis and coma. Occasionally, neurologic signs are diffuse rather than focal.
Workup
In a patient with steadily progressive focal neurologic deficits consistent with progressive multifocal leukoencephalopathy (PML), neuroimaging is indicated. The combination of a characteristic clinical picture and typical imaging findings supports a confident presumptive diagnosis of PML. [34]
Computed tomography or magnetic resonance imaging
With CT scan or MRI of the brain, single or multiple confluent lesions without mass effects are seen, most frequently in the parieto-occipital white matter. [1] Occasional infratentorial lesions are usually asymmetrical. The demyelinating plaques involve subcortical U fibers but tend to spare the cortical ribbons and deep gray matter structures; however, cases have been described that have involvement of deep gray matter. Subcortical gray matter or the spinal cord may be involved, but rarely. Gray matter involvement has a scalloped appearance.
PML sometimes can resemble lymphoma, toxoplasmosis, or HIV encephalitis. However, the absence of a mass effect or displacement of normal structures is more consistent with PML than these other disorders. [34] Rarely, PML can also present as a mass lesion with enhancement on postcontrast MRI scans. Magnetization transfer ratio (MTR) is typically low in PML cases compared with normal white matter and that of HIV-infected white matter without PML.
CT scan may show hypodense lesions. MRI scan is far more sensitive than CT scan. [37] On MRI, PML lesions characteristically are hypointense on T1-weighted images; this finding may be subtle but can help distinguish PML lesions from those of other diseases (eg, white matter lesions of HIV encephalitis). On T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences, PML lesions are characteristically hyperintense (see the image below). [34]
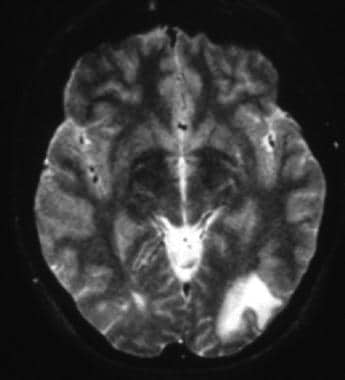 T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
Neuroimaging in patients with inflammatory PML may demonstrate atypical features, including a mass effect of the PML lesions with surrounding edema. Contrast enhancement, which is uncommon in classic PML and tends to be sparse when it does occur, may be striking in patients with inflammatory PML. [34] Any area of the white matter can be affected but is usually in the cerebellum.
Lumbar puncture
Cerebrospinal fluid (CSF) is usually normal, but protein levels may be elevated slightly. Normal CSF findings serve to rule out other etiologies. CSF pleocytosis can sometimes occur, but the cell count is usually less than 20/µL. JC virus culture in the CSF is usually unrevealing.
Polymerase chain reaction (PCR) of the CSF has been shown to be highly specific (92-99%) and sensitive (74-93%) for the detection of JC virus in patients with PML. [38] False negatives may be due to the low viral DNA in the CSF, storage of the specimen, low volume of the specimen, and loss of DNA during concentration. The false-positive rate has been reported to be around 2%. Measuring CSF JC virus DNA load is a reliable marker of disease activity in patients receiving HAART and has a potential use in drug trials. Conceivably, this test could eliminate the need for brain biopsy. However, the detection of JC virus in CSF may be less likely in patients with inflammatory PML. [34] If PML is suspected, even though the initial JC virus PCR is negative, the recommendation is to repeat the spinal fluid analysis.
Brain Biopsy
Brain biopsy has a sensitivity of 74–92% and a specificity of 92–100% in progressive multifocal leukoencephalopathy (PML). Mild cortical atrophy may be seen on biopsy specimens. Immunohistochemistry or in situ hybridization is the best method to confirm JC virus in the biopsy specimen.
Multiple demyelinative foci may be seen in the cerebral, cerebellar, and brainstem white matter and at the gray-white matter junction; in severe cases, such foci may be seen in the cortical gray matter. Foci may become confluent (see the images below). Oligodendrocytes at the gray-white junction are the most common sites of infection. JC virus infects not just the oligodendrocytes and astrocytes but also the granule cells of the cerebellum.
 This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
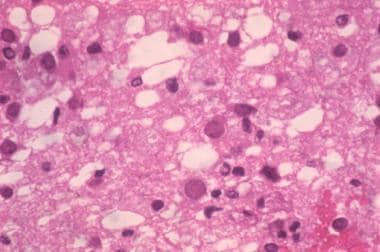 Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
Perivascular inflammatory infiltrates are observed. Necrotic and cystic lesions may be present but are rare.
Nuclear inclusions may be seen in large ballooned oligodendrocytes and rarely in astrocytes, both of which show bizarre-looking nuclei. The inclusions contain viruses as identified by electron microscopy and immunohistochemistry. Because of the cellular atypia, PML can be occasionally misdiagnosed as glioma. [39]
Diagnosis
In April 2013, the Neuroinfectious Disease Section of the American Academy of Neurology released a consensus statement on the diagnostic criteria of progressive multifocal leukoencephalopathy (PML) that stated that PML can be diagnosed either histopathologically or with clinical, radiologic, and virologic evidence. [40]
The diagnosis of Progressive Multifocal Leukoencephalopathy (PML) is confirmed through a combination of histopathologic features—demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei—along with the detection of JC virus via electron microscopy, immunohistochemistry, or PCR. Alternatively, a diagnosis can be made with compatible clinical symptoms, classic radiologic findings on MRI, and a positive CSF JC virus PCR, which together provide sufficient evidence for an unequivocal diagnosis of PML. [40]
However, the absence of clinical or radiologic features despite a positive CSF JC virus PCR, when using clinical criteria, or the inability to demonstrate JC virus despite classic histopathologic features, when using histopathologic criteria, categorizes the diagnosis as probable PML. Patients with probable PML should be managed as if they have confirmed PML. [40]
PML can present with a range of initial symptoms including cognitive and neuropsychiatric changes, visual and motor impairments, sensory disturbances, coordination issues, and seizures. [17] MRI scans typically show T2 hyperintense lesions that blur the boundaries between white and gray matter, with signal intensity varying according to the disease stage.
CSF analysis plays a crucial role, with JCV DNA detection by PCR confirming a definitive diagnosis of PML. However, the sensitivity of PCR assays can vary, and low viral loads may result in false negatives, necessitating repeated tests or the use of more sensitive methods such as digital droplet PCR. The future detection of anti-JCV-specific immunoglobulin G antibodies in CSF may also become a valuable diagnostic tool.
Despite clear diagnostic criteria, the identification of PML is often delayed due to its rarity and nonspecific symptoms, highlighting the need for increased awareness and education among healthcare providers. Implementing enhanced MRI screening protocols, particularly for high-risk individuals such as those undergoing natalizumab therapy, could lead to earlier detection and improved outcomes. The ongoing development of more sensitive diagnostic methods and increased access to these technologies are essential for reducing diagnostic delays and potentially updating current criteria to more accurately reflect the spectrum of JCV-associated diseases.
The diagnosis of PML remains less certain in the possible PML category.
The following tables demonstrate these criteria:
Table 1. Establishing the diagnosis with clinical, radiographic, and laboratory data. (Open Table in a new window)
Certainty of PML diagnosis |
Compatible Clinical Features |
Compatible Image Findings |
CSF JCV PCR |
Definite |
+ |
+ |
+ |
Probable |
+ |
- |
+ |
- |
+ |
+ |
|
Possible |
+ |
+ |
- /or not done |
- |
- |
+ |
|
Not PML |
- |
- |
- |
+ |
- |
- |
|
- |
+ |
- |
Table 2. Establishing the diagnosis with histopathology. (Open Table in a new window)
Certainty of PML diagnosis |
Classic Histopathologic Triad* |
Immunohistochemistry or Electron Microscopy |
Tissue JCV PCR |
Definite |
+ |
+ |
+ |
+ |
-/or not done |
+ |
|
+ |
+ |
-/or not done |
|
Probable** |
+ |
- |
-/or not done |
Possible |
- |
+ |
-/or not done |
Not PML |
- |
- |
-/or not done |
*Histopathologic triad: demyelination, bizarre astrocytes and enlarged oligodendroglial nuclei.
**Presence of clinical and radiologic evidence not resulting from other disease processes increases the certainty of this category to definite.
Treatment and Management
The treatment of progressive multifocal leukoencephalopathy (PML) primarily focuses on restoring JCV-specific immunity, crucial for managing the disease. This involves initiating combination antiretroviral therapy (cART) in HIV-infected patients or discontinuing immunosuppressive therapies that may have triggered PML. The effectiveness of interventions such as plasma exchange or immune adsorption in natalizumab-associated PML is still unconfirmed, and there is a risk of inducing PML-immune reconstitution inflammatory syndrome (PML-IRIS). [17]
For managing PML-IRIS, corticosteroids are cautiously administered to control the intense immune response, though the optimal type, dose, and duration of treatment remain undefined. Other therapies like the CCR5-blocking agent maraviroc have not shown convincing benefits in clinical trials. [17]
Immune checkpoint inhibitors
Pembrolizumab, a humanized monoclonal antibody targeting the programmed cell death protein-1 (PD-1) on lymphocytes, has emerged as a promising treatment for Progressive Multifocal Leukoencephalopathy (PML). Developed initially for cancer treatment, this immune checkpoint inhibitor has shown encouraging results in a pilot trial involving PML patients. [41] In this trial, eight patients with progressing PML received pembrolizumab (2 mg/kg) every 4–6 weeks for up to three doses, with five of the eight patients experiencing clinical improvement or stabilization. [42]
Further evaluation of immune checkpoint inhibitors (ICIs) in PML was conducted by Bouzmaza et al., who studied 79 patients and reported a one-year survival rate of 51.9%. [43] The study highlighted a significant correlation between MRI contrast enhancement and increased survival, as well as a reduction in JC polyomavirus DNA load among survivors. However, the treatment was not without risks; 19% of the patients developed PML-Immune Reconstitution Inflammatory Syndrome (PML-IRIS), and 30.4% experienced adverse events severe enough that some discontinued treatment. These findings emphasize the need for personalized treatment approaches to effectively manage the variable outcomes and high risk of complications.
The broader application of ICIs in PML, particularly in cases related to HIV where antiretroviral therapy (ART) may be ineffective, is under investigation. Despite the general exclusion of people living with HIV (PLWH) from ICI trials, emerging data suggest that ICIs are well-tolerated in HIV-infected populations. Current research indicates that ICIs can potentially reinvigorate the immune system to enhance antiviral responses in PML. However, the risk of ICIs inducing PML-IRIS or general IRIS remains a significant concern, influencing treatment timing and patient selection.
One of the challenges in deploying ICIs for PML treatment is the lack of reliable biomarkers to predict responses. Observations have shown that severe lymphopenia and the absence of detectable anti-JCV activity correlate with poor responses to ICIs. Investigating ICI-responsive T cell subpopulations could provide insights into these variable outcomes and help refine treatment strategies. Formal, prospective studies are crucial to evaluate the role of ICIs in advanced treatment regimens for HIV-related PML, aiming to establish more effective therapies for this severe disease.
Antiretroviral therapy and other experimental treatments
Other than this new, experimental treatment for PML, the principal approach is antiretroviral therapy. [34] Treatment guidelines for PML recommend (1) starting antiretroviral therapy immediately in patients with PML who are not on therapy and (2) optimizing the antiretroviral regimen for virologic suppression in patients who are receiving antiretroviral therapy but who remain HIV-viremic because of antiretroviral resistance.
Current research is exploring various treatments targeting different stages of the JCV replication cycle, with several interventional studies listed on ClinicalTrials.gov. However, no antiviral treatments have shown a significant impact on survival or neurological outcomes in PML patients. Experimental treatments, including recombinant interleukin therapies and checkpoint inhibitors, are being investigated but have shown variable results. [17]
One promising strategy is passive immunization using JCV or BK polyomavirus-specific T cells, which have demonstrated potential benefits in small retrospective studies. An ongoing pilot study is assessing the safety and feasibility of this approach, though definitive proof of efficacy is pending. [17]
The use of drugs that block the serotonergic 5HT2a receptor (eg, olanzapine, ziprasidone, mirtazapine, cyproheptadine, risperidone) has been suggested as treatment for PML, based on a report indicating that this receptor can serve as the cellular receptor for JCV. Only anecdotal reports of this approach are available, however, and its routine use is not considered justified. [34]
Mefloquine has been suggested as one of the options based on its in vitro activity against the JC virus, but a recent trial showed the lack of efficacy. [44] The current consensus is that interferon-alfa does not help in the treatment of PML, though some reports initially suggested its use.
Inhibition of retrograde transport of the JCV to the endoplasmic reticulum was investigated by two compounds: retro-2cycl and brefeldin A. Both retro-2cycl and brefeldin A were shown to inhibit JCV infection in vitro, and retro-2cycl was also shown to inhibit infectious spread in cultured cells. [45] However, these compounds remain investigational and so far no studies show their efficacy or safety in humans.
In some individuals, especially those with very low CD4+ counts, worsening of PML or new-onset PML can be observed after the initiation of highly active antiretroviral therapy (HAART). This is thought to be secondary to immune reconstitution inflammatory syndrome (IRIS). IRIS is considered as a paradoxical deterioration of a preexisting infection that is related to the recovery of the immune system. It is suggested to occur due to an imbalance of CD8+/CD4+ T cells.
Anecdotal case reports of use of pulsed methylprednisolone have shown rapid improvement in IRIS-associated PML. [46] Treatment guidelines consider the use of corticosteroids justified in this setting (B rating). [34]
Treatment trials have been conducted with cidofovir (used in AIDS patients with CMV), [47] topotecan (topoisomerase inhibitor), and interferon-alfa. [48] Results have been inconclusive, however, and treatment guidelines do not recommend the use of any of those agents. [34]
Cytosine arabinoside at 2 mg/kg/day for 5 days showed a 30% response rate in one study with patients (non-AIDS–related PML ) in whom an 85% mortality rate was expected in 1 year. [49] Cytosine arabinoside, however, failed in AIDS patients with PML.
Passive and active immunization against JCV infection and PML is a potential approach that is being considered for future evaluation. [45]
-
T2-weighted MRI shows left occipital hyperintense white matter changes with the lesion margin reaching the cortex.
-
This sliced fixed brain shows multiple isolated or confluent gray demyelinative foci. Atrophy may be present. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.
-
Microscopically, multiple demyelinative foci are detected. The microscopic hallmark of the disease is intranuclear basophilic or eosinophilic inclusions within the swollen nuclei of oligodendrocytes, often at the periphery of lesions. Large, occasionally multinucleated astrocytes with prominent processes are another characteristic feature. Contributed by Dr Beth Levy, Saint Louis University School of Medicine, St Louis, Missouri.

