Background
Acute disseminated encephalomyelitis (ADEM) is an acquired inflammatory demyelinating syndrome that predominately affects the white matter of the brain and spinal cord in a multifocal distribution. Clinically, ADEM manifests as subacute-onset of encephalopathy associated with polyfocal neurologic signs and sytmpoms. [1, 2, 3] ADEM may mimic other acute demyelinating syndromes (ADS) of childhood, including multiple sclerosis (MS); however, ADEM is typically distinguishable from alternative diagnoses on the basis of clinical features and findings on neuroimaging and laboratory investigations. With the discovery of antibodies targeting myelin oligodendrocyte glycoprotein (MOG), ADEM is one of the most common phenotypes of MOG antibody disease (MOGAD) in children. [4]
In more than half of cases, the onset of ADEM occurs in the wake of a prodromal illness that may include fever, malaise, headache, respiratory, and/or gastrointestinal symptoms. ADEM following vaccination is rare, accounting for < 5% of cases. [5] ADEM is typically a monophasic disease of prepubertal children, whereas, MS is typically a chronic relapsing and remitting disease of young adults. Unlike MS, children with ADEM do not typically exhibit intrathecally unique oligoclonal bands. If oligoclonal bands are present, a mirrored pattern (eg, an equal number of bands in the blood and CSF) is usually seen. In addition, MOG antibodies are present in more than half of pediatric ADEM cases and the presence of these antibodies at reliable titers argues against a diagnosis of MS. [6]
Susceptibility to ADEM is likely the product of multiple factors, including a complex interrelationship of genetics and exposure to infectious agents and other environmental factors. Many cases of ADEM possibly occur as the result of an inflammatory response provoked by infectious trigger. Typically, the manifestations of ADEM occur quickly after this systemic illness and are monophasic. In a minority of cases, patients with ADEM experience recurrences, termed multiphasic ADEM. Unlike ADEM, MS typically manifests as a relapsing-remitting illness in ensuing adolescence or young adulthood, a significant and unexplained latency of effect with apparent permanency of immune dysregulation. MS clinical attacks occur without a febrile prodrome. Uncommonly, MS develops in prepubertal individuals and ADEM develops in post-pubertal individuals. In very rare instances, individuals manifest prepubertal ADEM and, after long latency, MS in adolescence.
Pathophysiology
The distinction between multiple sclerosis (MS) and acute disseminated encephalomyelitis (ADEM) may prove challenging initially. The prominence of perivenular demyelination is considered the pathological signature of ADEM, whereas, the hallmark of MS is sharply demarcated confluent demyelinating plaques. In addition to perivenous inflammation and demyelination, cortical microglial pathology, microscopic hemorrhage, and lymphocytic meningeal infiltration may also be noted. [7] Of note, multifocal cortical microglial activation involving nondemyelinated cortex has been noted in ADEM and has been hypothesized to represent the pathological substrate of encephalopathy classically noted in ADEM and not in MS. [7]
Basic studies have shown that, in the earliest stages of inflammation, both MS and ADEM are likely to be mediated by stimulated clones of T-helper cells sensitized to autoantigens such as myelin proteins. Some studies have even identified serum autoantibodies to various myelin proteins that help to differentiate ADEM from MS. In particular, ADEM appears to be characterized by class-switched IgG autoantibodies, supporting the hypothesis of an antigen-driven immune response in ADEM cases, whereas, MS cases are characterized by serum IgM autoantibodies. [8] The complex ensuing inflammatory cascade entails the local action of cytokines and chemokines as well as lymphokine-induced chemotaxis of other cellular mediators of inflammation (eg, other T-cell lines, B cells, microglia, phagocytes).
Pathogenic differences of MS and ADEM are likely to arise in part because of differences in details concerning pro-inflammatory and anti-inflammatory cytokines and chemokines. Interleukin (IL)–1beta, Il-2, IL-4, IL-5, IL-6, IL-8, IL-10, interferon (IFN)–gamma, tumor necrosis factor-alpha, and macrophage inflammatory protein-1-beta are significantly elevated in CSF compared with the CSF of controls. Granulocyte colony-stimulating factor shows a particularly striking elevation at as much as 38-fold greater concentration than is found in the CSF from control subjects. Elevations of IFN-gamma, IL-6, and IL-8 have been significantly correlated with CSF cell counts and protein concentration in individuals with ADEM. The pattern of cytokine elevation suggests that ADEM involves activation of macrophages, microglial cells, and various Th (T helper)–1 and Th2 cells. [9]
Additionally, in 2006, Franciotta et al demonstrated that adults with ADEM have higher CSF concentrations of chemokines that recruit or activate neutrophils (CXL1 and CXL7), monocytes (CCL3 and CCL5), Th1 cells (CXCL10), and Th2 cells (CCL1, CCL17, and CCL22) than healthy normal controls. [10] Moreover, ADEM-associated concentrations of certain of these neutrophils (CXL7 neutrophil activator and the CL1, CCL17, and CCL22 Th2 activators) are higher in the CSF from individuals with ADEM than those with MS. On the other hand, CSF concentrations of the chemokine CCL11 is lower in adults with MS than in the CSF from adults with ADEM or in normal controls.
Disturbance of the blood–brain barrier is likely to be an important event. It is now recognized that ADEM is one of the most common manifesting phenotypes of MOG antibody disease (MOGAD) in children. The pathobiology of MOGAD has become more defined over time and is hypothesized to have an "outside-in" approach, with initial activation of MOG-specific T cells potentially via MOG-peptide presentation, bystander activation, or molecular mimicry. These peripherally activated T cells and MOG antibodies ultimately cross the blood–brain barrier at the time of the attack. Similar to noted general pathology of ADEM above, MOGAD lesions show a perivenous confluent pattern on histology. [11] Unlike aquaporin-4 IgG, it remains unclear if MOG IgG has a direct pathogenic role in MOGAD, if it contributes secondarily, or if it is only a marker of the underlying pathobiology.
Epidemiology
Frequency
Although considered a rare illness, acute disseminated encephalomyelitis (ADEM) affects an estimated 0.07 to 0.9 per 100,000 children annually. [12, 13]
The Canadian Pediatric Surveillance Program for acute demyelinating syndromes (ADS) in individuals younger than 18 years found that in 219 identified Canadian cases, ADEM represented 22% of diagnoses. This study generated an estimate of Canadian disease incidence of 0.9 per 100,000 for ADS, while cases of ADEM manifested an annual incidence of 0.2/100,000. [14] Higher incidence rates have been reported in San Diego County at 0.4/100,000 per year. [15]
Whether the increasing incidence of MS at increasing distance from the equator is also true of ADEM is unknown. The seasonal incidence of ADEM within North America peaks in the winter and spring months. [15, 16] Some severe forms of ADEM, such as those that occur in the wake of measles and the severe hemorrhagic variant called acute hemorrhagic leukoencephalopathy (AHLE) are probably less commonly encountered than they were prior to widespread immunization against measles and other formerly common and potentially serious illnesses that may serve as triggers for ADEM/AHLE.
Few studies have provided incidence data from other countries, thus little is known about occurrence throughout the world. Data from Germany quote an incidence rate of 0.07 per 100,000, [17] while data from Japan show an incidence of 0.64 per 100,000 per year. [18] Genetic factors, prevalence of infectious pathogens, immunization status, degree of skin pigmentation, diet, and other factors may influence risk.
Mortality/morbidity
Although older studies suggest a 10% mortality rate, the data upon which such estimates were based were obtained in epochs during which measles was prevalent, techniques for intensive care were comparatively primitive, and anti-inflammatory therapies were inadequate. Formerly, deaths occurred in patients with acute hemorrhagic leukoencephalitis (AHLE), a severe ADEM variant, which has become less common since children have received immunization to many common childhood illnesses.
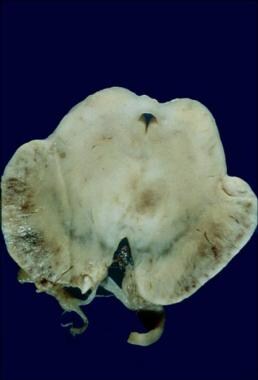
Current acute mortality rates are probably less than 2%, typically consisting of cases with fulminant cervical transverse myelitis or brain swelling. Children younger than 2 years are particularly subject to such severe presentations. [1] See the image below.
Morbidity chiefly includes visual, motor, autonomic, and behavioral/intellectual deficits and epilepsy. Based upon existing data, full recovery occurs in approximately 57–92% of patients. Residual focal neurologic deficits remain in 4–30%. [16, 19, 20, 21, 22, 23, 15, 24, 25] Neurocognitive deficits after acute demyelinating syndromes are receiving more attention, as even children having an apparent full recovery after ADEM have demonstrated subtle deficits on formal neuropsychological testing years after the sentinel event. [26] Additionally, these intellectual and behavioral impairments are variable depending on age of the child at the time of disease onset. There may be a greater impact upon behavior and intelligence in those with an ADEM-onset of younger than 5 years. [27]
Though uncommon, adult-onset ADEM, when correctly diagnosed, appears to have similar outcomes and a typically favorable prognosis. [28]
Race
The scientifically imprecise concept of race does not lend itself readily to discussions of ADEM. ADEM is found in all ethnic groups and races; referral bias complicates an assessment of relative prevalence.
Regardless of race, the degree of skin pigmentation directly influences vitamin D status in any given individual. [29] Several studies have implicated vitamin D deficiency as a contributing risk factor for MS, [30, 31, 32] though these studies have not been performed within ADEM cohorts.
Sex
Though no clear gender predominance has been identified, a handful of ADEM cohorts have reported a slightly male predominance. [14, 16, 25] These data are in opposition to the strong female preponderance noted within MS.
Age
More than 80% of childhood cases occur in patients younger than 10 years, with a mean age range of 5 to 8 years. [19, 24, 33] . Somewhat less than 20% of cases occur in the second decade of life. Incidence in adulthood is unclear, accounting for less than 3% of the reported cases; however, diagnostic overlap with MS may lead to underestimation of the prevalence in adults. [28] Adult-onset cases of particular severity are recognized upon the basis of biopsy. These cases may manifest very large white matter lesions that involve, as is the case with childhood-onset cases, the gray-white junction of forebrain.
Prognosis
The outlook for recovery in acute disseminated encephalomyelitis (ADEM) is generally excellent. Although some older series suggest up to a 10% mortality rate, other studies present mortalities of 0% in treated ADEM cases. [19, 20, 24, 25] Degree of recovery appears to be unrelated to severity of illness. Complete recovery may be observed even in children who become blind, comatose, and quadriparetic. Recovery is poorest in children younger than 2 years, patients with myelitis, and those who have significant edema of the brain or spinal cord. In other cases of ADEM, modest visual or motor deficits may persist, as may sphincter abnormalities in patients with spinal cord disease. Disturbances of mood and personality may outlast motor deficits, but they may also wane over ensuing months. The presence of myelin oligodendrocyte glycoprotein (MOG) antibodies in the setting of ADEM suggest a higher risk of MOG antibody disease (MOGAD) relapse and a greater risk of post-ADEM seizures. [34] Most patients with ADEM can look forward to complete recovery or the persistence of only mild deficits. This excellent outlook even applies to patients who experienced a global low state of function during the acute illness.
Whether any available form of treatment has a favorable effect on the time to maximal recovery or the risk for deficits is unknown. Exceptions to the excellent outlook are patients with transverse myelitis and infants younger than 2 years. Cord swelling may account for the high rate of residual paresis and the occasional death of patients with severe acute inflammatory myelitis. Prompt administration of high doses of corticosteroids can possibly improve the outlook for these patients, but no reliable data yet support this hypothesis.
Less than 10% of children with ADEM will experience a second event of demyelination > 3 months after the initial ADEM, [25] and the majority of these patients who relapse exhibit evidence of MOG IgG. [35] The presence of MOG antibodies, at reliable titers, argues against a future diagnosis of MS. [36]
-
Fatal case of ADEM involving the brainstem in a 13-month-old.
-
Typical childhood ADEM in 7-year-old. Note tendency to involve gray-white junction, the fact that the lesion margins are less well defined than typical MS plaques, and that the deep white matter lesions are not oriented perpendicularly to the ventricular surface as is typical in MS.
-
Typical adolescent multiple sclerosis findings on MRI. Note the tendency of lesions to exhibit sharp margins, to be elongated, to occur in deep white matter or corpus callosum sparing the cortical gray-white junction, and to be oriented perpendicularly to the ventricular surface.
-
Fifteen year old female diagnosed with multiple sclerosis. MRI is a sagittal FLAIR image demonstrating ovoid lesions that are perpendicular to the long axis of the corpus callosum. Juxtacortical lesions are also noted.
-
5 year old boy with subacute onset of encephalopathy, weakness, and visual changes. He was diagnosed with ADEM and was positive for serum MOG antibodies. MRI is an axial FLAIR image demonstrating hyperintensities throughout the subcortical white matter that are asymmetric in distribution, patchy, and large.
-
3 year old with ADEM (MOG+). MRI axial FLAIR imaging shows hyperintense lesions within the bilateral putamen and thalami (right greater than left).