Background
Venous thromboembolism (VTE, including deep vein thrombosis [DVT] and pulmonary embolism [PE]) in surgical patients undergoing general anesthesia has been extensively studied. The risk of VTE remains high for up to 2 months after noncancer general surgery. [1]
Fatal PE rates range from 0.1% to 0.8% for all patients [2, 3] and may be as high as 7% for patients undergoing surgery for fractured hips. [4] A study of patients with pelvic or lower-extremity fracture (N = 3295) by Pan et al found a 2.08% incidence of PE in patients with below-knee DVT and a 3.17% incidence in patients with above-knee DVT. [5] In many patients who undergo foot and ankle surgery, DVT may develop without clinically apparent symptoms or signs. [6]
Many different forms of therapy have been evaluated in this group. Studies of pneumatic compression in cardiac surgery and neurosurgical patients have shown a distinct improvement in the incidence of DVT without the added risk of bleeding. [7, 8] However, the effect is less impressive in higher-risk patients, and compliance can be difficult.
The timing and duration of pharmacologic prophylaxis have also been determined to exert a significant effect the development of DVT. Early prophylaxis in surgical patients with low-molecular-weight heparin (LMWH) has been associated with significant reductions in postoperative venous thrombosis. A study by Hull et al found that initiation of therapy within 8 hours of surgery had the greatest effect. [9]
The ninth edition of the clinical practice guidelines for prevention of VTE from the American College of Chest Physicians (ACCP) recommended that LMWH be given to patients undergoing major orthopedic procedures at least 12 hours preoperatively or postoperatively. [10] The 2016 updates for the 10th edition of the ACCP guidelines did not contain significant changes with regard to VTE prophylaxis (as distinct from treatment) in orthopedic surgery. [11]
In August 2024, updated European guidelines for prophylaxis of VTE in nonambulatory orthopedic surgery were published. [12]
For more information, see Deep Venous Thrombosis and Pulmonary Embolism.
Mechanical Methods
Mechanical methods have been shown to be a useful adjunct to anticoagulation therapy in reducing the incidence of DVT. Modalities include passive devices, such as knee- or thigh-high graduated compression (elastic) stockings (GCS) [13] ; active (external pneumatic compress or intermittent pneumatic compression [IPC]) devices [14] ; or venous foot pumps (VFP). [15]
A 2012 systematic review of randomized, controlled trials found that knee- and thigh-high GCS do not significantly differ in their effectiveness in reducing the incidence of DVT in hospitalized patients. Ease of use, patient compliance, and cost influence which type of stocking is used in clinical practice. [16]
In a study of the efficacy of IPC in multiple postoperative patient groups versus no use of prophylaxis, Urbankova et al reported that the incidence of DVT was reduced by 60%. [17] However, the use of mechanical means of prophylaxis alone is not effective in moderate or high-risk cases.
IPC devices are designed to decrease venous stasis, improve blood flow velocity, and increase the level of circulating fibrinolysins. These devices have the advantage of requiring no monitoring, with no increase in bleeding. Generally, they are well tolerated. Various forms of IPC devices are available, and they can be applied to the foot, calf, or thigh. A study comparing asymmetrical with circumferential IPC devices following total knee replacement (TKR) seemed to support use of the asymmetrical device. [18]
Patient compliance is an issue with IPC devices, and efficacy is dependent on the time of use. Evidence from clinical trials has shown that although the rate of distal thrombi is reduced significantly, that of proximal thrombi is not. This finding may lead to a false sense of security because although the total number of deep venous thrombi may be similar to the numbers observed with pharmacologic prophylaxis, the proportion of the relatively more dangerous proximal clots is increased (see Table 1 below).
Table 1. Frequency of Thrombi at Different Sites With Intermittent Pneumatic Compression vs Warfarin (Open Table in a new window)
Thrombi |
Warfarin (n = 72) |
IPC (n = 67) |
Iliac and femoral |
5 |
14 |
Calf, popliteal, plantar |
10 |
2 |
Total |
15 |
16 |
Although all three types of mechanical compression reduce the incidence of DVT to less than that found when prophylaxis is absent, these modalities are generally less effective at producing such reductions than pharmacologic methods are. Shorter hospital stays make the use of mechanical methods alone ineffective in preventing DVT in the critical weeks after joint replacement. No mechanical prophylaxis method has been shown to reduce the risk of PE or death. The use of IPC devices is therefore recommended primarily as an adjunct to anticoagulant-based prophylaxis or in patients who are at high risk of bleeding.
Pharmacologic Methods
Many pharmacologic agents are currently available to prevent thrombosis. Agents that retard or inhibit the process belong under the general heading of anticoagulants. Agents that prevent the growth or formation of thrombi are properly termed antithrombotics and include anticoagulants and antiplatelet drugs, whereas thrombolytic drugs lyse existing thrombi. For the importance of prevention, see Hull and Pineo's 1998 study. [19, 20, 21]
In a systematic review and meta-analysis (61 studies) evaluating the efficacy of 11 anticoagulants for preventing VTE after total hip arthroplasty (THA) or total knee arthroplasy (TKA), Huang et al found apixaban, edoxaban, fondaparinux, rivaroxaban, and darexaban to be the most efficacious agents. [22]
There is some evidence to suggest that statins have the potential to reduce recurrent events in patients with VTE. [23] One systematic review and meta-analysis (5 studies: 2 retrospective cohorts, 3 randomized controlled studies [RCTs]) suggested that statins may reduce postthrombotic syndrome after DVT; however, meta-analysis of the RCTs did not confirm any risk reduction. [24]
Platelet-active drugs
Platelet-active drugs such as aspirin or cyclooxygenase (COX)-1 inhibitors have been used to prevent thrombosis. [25] Aspirin is effective as a platelet inhibitor at very low dosages (50-100 mg/day). This dosage is significantly less than that necessary to produce an anti-inflammatory effect. However, a meta-analysis of the effect of aspirin following total hip replacement (THR) completed in 1994 had equivocal results. [26, 27]
A large study performed in Europe, the Pulmonary Embolism Prevention (PEP) study, found that the overall DVT rate was decreased 30% with low-dose aspirin as compared with placebo, and the overall pulmonary thrombosis rate was decreased by 40%. This trial included 13,356 patients with hip fractures and 4088 patients who underwent THR. [27] Aspirin at 160 mg/day was compared with placebo and evaluated at day 35. Approximately 40% of the patients also were given low-density heparin or LMWH.
In a concomitant study of the 4088 patients who underwent THR, a 25% reduction of DVT was observed in comparison with the placebo control, but no decrease was noted in the rate of PE. [27] This trial did not show a clear benefit to using aspirin as the primary method of venous prophylaxis in patients undergoing either total hip or total knee surgery.
The Seventh ACCP Conference did not recommend the use of aspirin alone as a prophylactic agent for any patient group, on the grounds that aspirin was less effective than other options. However, reports by Lotke and Lonner [28] and by Berend and Lombardi [29] suggested that the use of aspirin combined with optimally used IPC devices may be effective in some circumstances in preventing fatal PE.
Since these studies, the use of aspirin for prophylaxis has been evaluated further. A systematic review of eight studies (N = 43,012) by Mistry et al addressed the use of aspirin as thromboprophylaxis after knee and hip arthroplasty. [30] Overall, only 283 (0.66%) of the patients given aspirin had symptomatic DVT. Aspirin was found to be cost-effective, to have a good side-effect profile, and to have a lower rate of complications (eg, bleeding and wound oozing) than anticoagulants.
In a meta-analysis of 13 randomized controlled trials (N = 20,115), Haykal et al assessed the clinical efficacy and safety of aspirin against those of placebo and anticoagulants in patients undergoing knee or hip arthroplasty. [31, 32] The primary outcome was VTE incidence; secondary outcomes included any bleeding, major bleeding, and death. No significant differences among the three groups were noted with regard to the secondary outcomes. Aspirin was associated with a lower VTE incidence than anticoagulants, but the difference was not statistically significant. However, it was associated with a significantly lower incidence of VTE in comparison with placebo.
In a systematic review and meta-analysis of six studies (N = 4460) comparing the clinical effects of LMWH and aspirin with regard to DVT after orthopedic surgery, Chen and Hu found that LMWH was associated with a significantly lower incidence of DVT, though the incidence of postoperative bleeding did not differ between the two groups. [33]
In a study comparing aspirin with LMWH for thromboprophylaxis in patients who had either an operatively treated extremity fracture or any pelvic or acetabular fracture, O'Toole et al found aspirin thromboprophylaxis to be noninferior to LMWH prophylaxis for preventing mortality and to be associated with low rates of DVT and PE. [34]
An analysis of VTE trends after hip arthroplasty in the United States from 2011 to 2019 found that 90-day VTE rates after the procedure declined significantly over this period; it also found that the use of aspirin, direct factor Xa inhibitors, and direct thrombin inhibitors increased, whereas the use of other nonaspirin anticoagulants decreased. [35]
Coumarins
Coumarins are a class of oral anticoagulant drugs, which act as antagonists to vitamin K. The mechanism of action is to interfere with the interaction between vitamin K and coagulation factors II, VII, IX, and X. Vitamin K acts as a cofactor at these levels. Coumarins produce their anticoagulant effect by inhibiting the carboxylation necessary for biologic activity.
Warfarin is a mixture of two isomers, the R and S forms, in roughly equal proportions. This agent is absorbed rapidly from the gastrointestinal (GI) tract and bound to plasma proteins. Although it has high bioavailability, warfarin requires 36-72 hours to reach a stable loading dose. The dose response in patients taking warfarin is variable, and it is influenced by various genetic and environmental factors. In addition, numerous drug interactions and disease states may affect its pharmacokinetics. Warfarin, therefore, requires continuous laboratory monitoring.
The effectiveness of warfarin anticoagulation is measured by determining the prothrombin time (PT) or protime against a standard control. The use of the international normalized ratio (INR) has supplanted the PT for hospital use. INR uses a standardized PT, which allows for comparisons between hospitals and laboratories.
For DVT prophylaxis, the optimal INR is between 2 and 3, with a target of 2.5. When used for DVT prophylaxis after THR, warfarin reduces total DVT by 60% and proximal DVT by 70%. Disadvantages of warfarin use include its long onset of action, the necessity to monitor INR values frequently to obtain a stable dosage, the long half-life that may require vitamin K reversal in incidents of hemorrhage, frequent drug and dietary interaction, and variable patient response. Hemorrhagic complications are reported in up to 3-5% of patients on warfarin prophylaxis.
If adjusted-dose warfarin is to be used, it is started the night before surgery and continued postoperatively during the discharge period. INR target levels are not usually reached until postoperative day 3.
Heparins
Standard unfractionated heparin (UFH) is recognized as an acceptable anticoagulant modality and has been used for this purpose in various forms since its discovery by McLean in 1916. UFH acts in conjunction with a circulating plasma cofactor, antithrombin (AT) III and, in its presence, catalyzes the inactivation of factors IIa, Xa, IXa, and XIIa.
By inactivating thrombin, heparin not only prevents fibrin formation but also inhibits thrombin-induced activation of factor V and factor VIII. Of these, factors IIa and Xa are most sensitive. Therefore, heparin has anticoagulant and antithrombotic properties.
Heparin is a heterogeneous mixture of molecules that contain a range of molecular weights of 3-30 kd, with an average of approximately 15 kd. Only one third of the heparin molecules have an active binding site for ATIII, and this fraction is responsible for most of the anticoagulant activity.
Heparin is effective when given by intravenous (IV) or subcutaneous (SC) administration but is inactivated in the GI tract. This agent has a rapid onset of action, its half-life is brief in comparison to warfarin, and it binds to platelets, endothelial cells, and macrophages in vivo. Therapeutic levels of heparin are measured by the activated partial thromboplastin time (aPTT). Because of the rapid clearance of heparin from the bloodstream, therapeutic levels (aPTT of 1.2-1.5 × control) are more likely achieved with continuous IV infusion.
Postoperative DVT prophylaxis with UFH is usually achieved by administering a bolus of 5000 U every 8 hours. This low-dose heparin regimen results in a 60-70% reduction of DVT and PE in low-risk or moderate-risk patients. However, this method is not as effective in patients who are at high risk for development of DVT or PE. In these patients, adjusted-dose heparin with aPTT monitoring is preferred to maintain the desired anticoagulant level. Studies have demonstrated a high hemorrhagic complication rate of 8-15% when this method is used for postoperative DVT prophylaxis.
Heparin overdosage is reversible with protamine sulfate, which itself is an anticoagulant. Each milligram of protamine sulfate can neutralize approximately 100 U of heparin activity. This agent must be administered very slowly by IV infusion over a 10-minute period in doses not to exceed 50 mg. Because heparin is rapidly cleared from the circulation, the amount of protamine required decreases rapidly as the time from initial heparin administration increases. The final dosage required is titrated according to coagulation studies.
Disadvantages of UFH therapy include the following:
-
Variable pharmacokinetics
-
Requirement for aPTT monitoring for adjusted-dose regimens
-
Short half-life and low bioavailability
-
Lack of an oral dosage form (though an oral form has been included in clinical trials)
In addition, a small percentage of patients (2-4%) are susceptible to the development of heparin-induced thrombocytopenia (HIT), which is an antibody-mediated adverse reaction that can cause venous and arterial thrombosis. HIT is heralded by an otherwise unexpected fall in platelet count of greater than 50% from previous levels. HIT can result in disseminated intravascular coagulation (DIC) and gangrene in severe cases. Treatment with danaparoid sodium or recombinant hirudin, such as lepirudin, may be effective in life-threatening cases.
A comparison study by McGarry et al of outcomes of thromboprophylaxis between an LMWH (enoxaparin) and UFH revealed a 74% lower incidence of VTE in the LMWH group. [36] No significant difference in side effects, deaths in the hospital, or economics was noted.
Low-molecular-weight heparins
LMWHs are manufactured when standard heparin is treated by various enzymatic or chemical methods to select those lower-molecular-weight moieties that contain the active ATIII binding site. The average molecular weight of fractionated heparin is 4.5 kd rather than the usual 15 kd. The molecular-weight threshold under which anti-factor Xa activity is maximized is 5.4 kd.
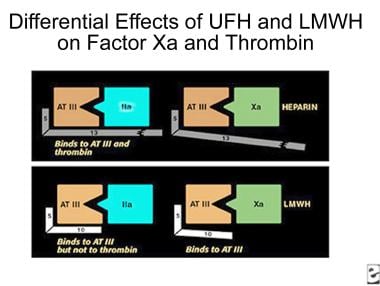
The polysaccharide side chain of the heparin molecule is decreased from 18 U to approximately 13 U. As the length of the side chain is decreased, the ability of the molecule to prolong the aPTT is lost, but the ability to complex with ATIII is retained. LMWHs do not require monitoring of either aPTT or INR (see the image below).
The pharmacologic effect of this transformation is to make the LMWH more bioavailable (~90%, compared with 29% for UFH) and to lengthen its half-life to 4 hours from 1 hour for UFH. LMWH also increases the activity ratio of anti-Xa to anti-IIa, resulting in increased antithrombotic activity.
In experimental models and animal studies, LMWH produces less microvascular bleeding than UFH, but this finding has not been duplicated in human trials. Compared with placebo, LMWHs produced a 70-80% risk reduction for DVT in numerous studies without an increase in major bleeding in high-risk orthopedic patients. Meta-analyses comparing various other methods of DVT prophylaxis, including low-dose UFH, adjusted-dose heparin, and warfarin, have demonstrated improvement in DVT prophylaxis without increase in hemorrhagic complications. [37, 38, 39]
Several LMWH medications are commercially available, including the following:
-
Enoxaparin - Dose 30 mg SC every 12 hours, starting 12-24 hours postoperatively; dosing may require adjustment in obese hospitalized patients [40]
-
Dalteparin - Dose 5000 IU SC daily (qd), starting 12-24 hours postoperatively [41]
-
Danaparoid - Dose 750 U SC every 12 hours, starting 12-24 hours postoperatively
-
Nadroparin - Dose 38 U/kg SC qd, starting 12-24 hours postoperatively
-
Tinzaparin [42] - Dose 75 U/kg/d SC, starting 12-24 hours postoperatively
-
Ardeparin - Knee surgery, dose 50 IU/kg SC every 12 hours, starting 12-24 hours postoperatively
Fondaparinux sodium, a synthetic pentasaccharide, selectively binds to ATIII and potentiates neutralization of factor Xa, inhibiting thrombin formation and thrombus development. [43] Fondaparinux acts rapidly but has a long half-life (18 hours). A dose of 2.5 mg SC daily can be started 6-8 hours postoperatively. Renal clearance requires a minimal kidney function of creatinine clearance (CrCl) of greater than 30 mL/min or a weight of over 110 lb. In a controlled study by Bauer et al, fondaparinux was more effective than enoxaparin in preventing DVT after TKR, but episodes of major bleeding were more frequent. [44] For a comparison between fondaparinux and enoxaparin, see Turpie et al. [45]
Combination therapies
Keeney and colleagues reported on the use of early mobilization with a combination of IPC and adjusted-dose short-duration warfarin in a group of patients undergoing 700 primary and revision total hip arthroplasties. [46] The investigators recorded a low incidence of clinical DVT (as measured by ultrasonography) on postoperative day 3 or 4 and of clinical DVT and PE within 90 days postoperatively. Further clinical investigations with larger numbers of patients are necessary to determine the optimal levels and duration of anticoagulation with the appropriate risk/benefit ratio.
Factor Xa inhibitors
Direct oral anticoagulants (DOACs) are increasingly being used as alternatives to LMWH for thromboprophylaxis in patients undergoing orthopedic surgery. A meta-analysis by Haykal et al found DOACs to be associated with significant reductions in major VTE and DVT as compared with LMWH. [47]
Large phase III clinical trials have described the use of the factor Xa inhibitor rivaroxaban for prevention of thromboembolism following total knee or total hip arthroplasty. Rivaroxaban is administered once daily and has shown significant superiority in preventing DVT, nonfatal PE, or death compared with SC enoxaparin following arthroplastic surgery. It was the first orally active direct inhibitor of coagulation factor Xa approved by the US Food and Drug Administration (FDA) and is indicated for prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery. [48, 49, 50, 51, 52, 53]
Rivaroxaban was compared with enoxaparin for acute DVT. Efficacy showed noninferiority to enoxaparin for short-term use. When used as continued treatment, rivaroxaban had superior efficacy (P< .001) compared with placebo. A study comparing rivaroxaban to warfarin for long-term efficacy is warranted. [54] Rivaroxaban was found to be equivalent to enoxaparin/vitamin K antagonist in the treatment of established DVT, also without an increase in bleeding complications.
Another factor Xa inhibitor, apixaban, was approved by the FDA in March 2014 for postoperative prophylaxis of DVT/PE following hip or knee arthroplasty. Apixaban compared favorably with enoxaparin in the prevention of DVT after hip or knee replacement without increased bleeding. [55, 56]
The factor Xa inhibitor edoxaban was approved by the FDA in January 2015 for treatment of DVT and PE in patients who have been initially treated with a parenteral anticoagulant for 5-10 days. It has also been used for prophylaxis. [57]
Betrixaban, another factor Xa inhibitor, was approved by the FDA in June 2017. It is indicated for prophylaxis of VTE in adults hospitalized for acute medical illness who are at risk for thromboembolic complications owing to moderate or severe restricted mobility and other risk factors that may cause VTE.
For more information, see Deep Venous Thrombosis and Pulmonary Embolism.
Direct thrombin inhibitors
In November 2015, the FDA approved dabigatran for prophylaxis of DVT and PE after hip replacement surgery. Approval was based on the RE-NOVATE and RE-NOVATE II trials. [58, 59] Dabigatran was found to have similar effectiveness and safety in preventing VTE following hip arthroplasty as compared with enoxaparin.
Deep Venous Thrombosis Prophylaxis Based on Risk Stratification
In separate studies, Rosendaal, Kearon, and Bulger et al analyzed the relative contribution of individual risk factors to the development of DVT. [60, 61, 62] When more than one risk factor is present, the risk is cumulative; however, no good model suggests how the individual risk factors interact. [63] Nonetheless, several attempts have been made to quantify the risk factors associated with VTE. [64] The use of a checklist to stratify patients and assign them to categories of relative propensity for DVT development is helpful in deciding on an appropriate treatment regimen (see below).
A list can be constructed by using the ACCP risk categories. [65] These figures include a list of the pertinent factors, which are arbitrarily assigned a risk level between 1 and 5. An individual aged 61-75 years is assigned 2 units; a person older than 75 years is assigned a score of 3, as is an individual with a previous history of thrombosis, inherited thrombophilia, antiphospholipid antibodies, or lupus anticoagulant.The total score is then added.
Risk factors are grouped according to severity and are added to produce an overall risk factor score, which corresponds to a low through a very high potential for DVT development.
In risk factor assessment, 1 point is assigned to each of the following:
-
Age 41-60 years
-
Minor surgery
-
History of major surgery within 1 month
-
Pregnancy or postpartum within 1 month
-
Varicose veins
-
Swelling of legs
-
Obesity (body mass index [BMI] >25 kg/m2)
-
Oral contraceptives, patch, or hormone replacement therapy
Each of the following risk factors is assigned 2 points:
-
Age older than 60 years
-
Malignancy or current chemotherapy or radiation therapy
-
Major surgery (>45 min)
-
Laparoscopic surgery (>45 min)
-
Confined to bed longer than 72 hours
-
Immobilizing cast shorter than 1 month
-
Central venous access for less than 1 month
-
Tourniquet time longer than 45 minutes
The following risk factors are assigned 3 points each:
-
Age older than 75 years
-
History of DVT or PE
-
Family history of thrombosis
-
Factor V Leiden/activated protein C resistance
-
Medical patient with risk factors of myocardial infarction, congestive heart failure, or chronic obstructive pulmonary disease
-
Congenital or acquired thrombophilia
Finally, 5 points are assigned to each of the following risk factors:
-
Major elective lower-extremity arthroplasty, TKR, THR
-
Hip, pelvis, or leg fracture within 1 month
-
Stroke within 1 month
-
Multiple trauma within 1 month
-
Acute spinal cord injury with paralysis within 1 month
These factors include those that diminish venous flow or return, increase viscosity, or alter mobility. Age is one of the most easily definable factors. [66] The risk of DVT increases in exponential fashion with increasing age (see the image below).
By using the risk criteria listed above, orthopedic patients can be categorized into four risk groups, ranging from low to very high (see Table 2 below). Appropriate methods of prophylaxis may be applied to each level.
Table 2. Deep Venous Thrombosis Risk Factor Scores (Open Table in a new window)
Risk Factor Score |
0-1 |
2 |
3-4 |
5+ |
DVT Incidence |
2% |
10-20% |
20-40% |
40-80% |
Risk level |
Low |
Moderate |
High |
Very high |
Low-risk patients have a score of 1 or less. These are individuals who are younger than 40 years who are undergoing a minor surgical procedure and have no additional risk factors. The risk of calf DVT in this group is estimated to be 2-5% without prophylaxis, and the risk of clinical pulmonary thrombosis is 0.2% No specific prophylaxis is required in this group other than early and aggressive mobilization.
Moderate-risk patients have a score of 2 or less. They are individuals in the above group who have additional risk factors or are aged 40-60 years who are undergoing nonmajor surgery and have no additional risk factors. Other risk factors are surgery requiring a tourniquet (eg, arthroscopy), lower-extremity fractures, cast immobilization, or spinal surgery.
Major surgery in patients younger than 40 years poses a moderate risk of DVT, which is estimated at 10-20%. The risk of clinical PE in this group is 1-2%. Successful prevention strategies in this group consist of low-dose UFH (LDUH; q12hr), LMWH (< 3400 U q24hr), and GCS or IPC.
High-risk patients have a score of 3 or 4 and include persons older than 60 years, as well as patients aged 40-60 years who have additional risk factors, such as previous VTE, malignancy, or hypercoagulability. The risk of calf DVT is estimated at 20-40% in this group, with clinical pulmonary embolism occurring in 2-4%. Successful prevention strategies in this group consist of LDUH (q8hr) and LMWH (>3400 U q24hr), with or without IPC.
The highest-risk patients have a score of 5 or greater; they are older than 40 years who have additional risk factors, who are undergoing hip or knee replacement surgery, or who have had hip fracture, open lower-leg fracture, multiple trauma, or spinal cord injury. Hip fracture patients have the highest risk of dying from a fatal PE. Additional risk factors may include a history of VTE, malignancy, or hypercoagulable state. These factors carry an estimated risk of calf DVT of 40-80% without prophylaxis, with clinical PE occurring in 4-10% and fatal PE in 0.2-5%.
Successful prevention strategies include LMWH (>3400 U q24hr), fondaparinux, and coumarins (INR 2-3). Dose-adjusted LDUH or LMWH may be used with or without IPC/GCS.
According to a meta-analysis (11 studies; N = 265,194; preexisting varicose veins n = 2188) by Westby et al, the presence of varicose veins increases the risk of VTE in patients undergoing major lower-limb arthroplasty, though additional study would be needed to determine whether such patients should undergo varicose vein surgery before the arthroplasty procedure. [67]
To see complete information on Deep Venous Thrombosis Risk Stratification, please go to the main article by clicking here.
European recommendations on perioperative VTE prophylaxis
Nonambulatory orthopedic surgery
In August 2024, updated European guidelines on perioperative prophylaxis of VTE in patients undergoing nonambulatory orthopedic surgery were published. [12] Recommendations included the following:
-
For evaluation of VTE and bleeding risks, routine patient-specific preoperative evaluation, based on procedure type and planned postoperative course (fast-track or standard), is suggested in preference to population-based preoperative evaluation
-
Routine fast-track procedures (including early ambulation and joint mobilization) are recommended over procedures timed on the basis of convenience
-
For patients undergoing low-VTE-risk procedures who do not have a personal high-risk factor for VTE, no pharmacologic VTE prophylaxis is suggested
-
For patients undergoing low-VTE-risk procedures who have a personal high-risk factor for VTE but no high risk of bleeding, pharmacologic VTE prophylaxis with either an LMWH or a DOAC is suggested in preference to no prophylaxis; no recommendation can be made either for or against aspirin
-
For patients undergoing low-VTE-risk procedures who have both a personal high-risk factor for VTE and a high risk of bleeding, mechanical VTE prophylaxis is suggested in preference to no prophylaxis
-
For patients undergoing high-VTE-risk procedures who have no high risk of bleeding, pharmacologic VTE prophylaxis with LMWH or DOAC is suggested in preference to no prophylaxis; no recommendation can be made either for or against aspirin
-
For patients undergoing high-VTE-risk procedures who have a high risk of bleeding, mechanical VTE prophylaxis is suggested in preference to pharmacologic prophylaxis
-
After THA, TKA, or hip fracture surgery, pharmacologic VTE prophylaxis is recommended over no prophylaxis
-
After fast-track THA, TKA, or hip fracture surgery, pharmacologic VTE prophylaxis with LMWH or DOAC is recommended over no prophylaxis
-
After fast-track THA or TKA, pharmacologic VTE prophylaxis with aspirin is recommended over no prophylaxis
-
After THA, TKA, or hip fracture surgery, pharmacologic VTE prophylaxis with LMWH or DOAC is recommended over no prophylaxis
-
After fast-track THA or TKA, pharmacologic VTE prophylaxis with LMWH, DOAC, or aspirin is recommended over no prophylaxis
Elderly patients
In September 2017, the European Society of Anesthesiology issued the following guidelines regarding prophylaxis for VTE in elderly patients undergoing surgery [68] :
-
The risk for postoperative VTE is increased in patients older than 70 years and in elderly patients presenting with comorbidities, such as cardiovascular disorders, malignancy, or renal insufficiency; therefore, risk stratification, correction of modifiable risks, and sustained perioperative thromboprophylaxis are essential in this patient population
-
Timing and dosing of pharmacoprophylaxis may be adopted from the younger population
-
Direct oral anticoagulants are effective and well tolerated in the elderly; statins may not replace pharmacologic thromboprophylaxis
-
Early mobilization and use of nonpharmacologic means of thromboprophylaxis should be exploited
-
In elderly patients, suggest identification of comorbidities increasing the risk for VTE (eg, congestive heart failure, pulmonary circulation disorder, renal failure, lymphoma, metastatic cancer, obesity, arthritis, post-menopausal estrogen therapy) and correction if present (eg, anemia, coagulopathy)
-
Suggest against bilateral knee replacement in elderly and frail patients
-
Suggest timing and dosing of pharmacologic VTE prophylaxis as in the younger population
-
In elderly patients with renal failure, low-dose UFH may be used or weight-adjusted dosing of LMWH
-
In the elderly, recommend careful prescription of postoperative VTE prophylaxis and early postoperative mobilization
-
Recommend multifaceted interventions for VTE prophylaxis in elderly and frail patients, including pneumatic compression devices, LMWH, and/or direct oral anticoagulants after knee or hip replacement
ACCP recommendations for major orthopedic surgery and knee arthroscopy
In 2012, the American College of Chest Physicians (ACCP) issued recommendations for VTE prevention in orthopedic surgery patients, based on the ninth edition of its evidence-based clinical practice guidelines for antithrombotic therapy and prevention of thrombosis. [10]
Recommendations for patients undergoing major orthopedic surgery (total hip arthroplasty [THA], total knee arthroplasty [TKA], or hip fracture surgery [HFS]) included the following [10] :
-
In patients underoing TKA or THA, LMWH, fondaparinux, apixaban, dabigatran, rivaroaxaban, LDUH, adjusted-dose vitamin K antagonist (VKA), aspirin, or an IPC device for at least 10-14 days is preferable to no prophylaxis
-
In patients underoing HFS, LMWH, fondaparinux, LDUH, adjusted-dose VKA, aspirin, or an IPC device for at least 10-14 days is preferable to no prophylaxis
-
In patients who receive LMWH, prophylaxis should be started at least 12 hours preoperatively or postoperatively
-
Regardless of concomitant IPC device use or duration of treatment, LMWH is favored over alternative recommended agents
-
Thromboprophylaxis should be extended in the outpatient period for up to 35 days from the day of surgery
-
During the hospital stay, dual prophylaxis with and IPC device and an antithrombotic agent is suggested
-
In patients who are at increased risk for bleeding, an IPC device or no prophylaxis is favored over pharmacologic prophylaxis
-
In patients who refuse or will not cooperate with injections or an IPC device, apixaban or dabigatran (or, if these are unavailable, rivaroxaban or adjusted-dose VKA) is recommended
-
In patients who are at increased risk for bleeding or in whom both mechanical and pharmacologic prophylaxis are contraindicated, placement of an inferior vena cava filter is not recommended
-
In patients who are asymptomatic after surgery, Doppler ultrasound screening before discharge is not recommended
In patients undergoing knee arthroscopy who do not have a prior history of VTE, no thromboprophylaxis was recommended. [10]
The 2016 updates for the 10th edition of the ACCP guidelines did not contain significant changes with regard to VTE prophylaxis (as distinct from treatment) in orthopedic surgery. [11]
Elective spine surgery
For patients who have no additional risk factors, antithrombotic prophylaxis following elective spine surgery is not recommended. Patients at high risk for developing postoperative VTE may be treated with LDUH, LMWH, or perioperative IPC. Multiple risk factors may require the combined use of mechanical and pharmacologic measures.
Epstein reported a 2.8% incidence of DVT and a 0.7% incidence of PE in 139 patients following multilevel lumbar spine surgery treated with IPC and early mobilization. [69] For prevention of thromboembolism in spinal cord injuries, see the recommendations of the Consortium for Spinal Cord Medicine. [70]
Anesthesia
Anticoagulant prophylaxis should be used with caution in patients receiving spinal or indwelling catheter epidural anesthesia. Although the risk of spinal hematoma is very small (0.0025% with spinal anesthesia and 0.03% with epidural anesthesia), care should be taken to delay the initiation of thromboprophylaxis for at least 2 hours after catheter removal. Patients with known bleeding disorders should not receive preoperative prophylaxis if they are to receive spinal anesthesia. In cases of traumatic spinal tap with bloody spinal fluid, postoperative administration of thromboprophylaxis should be done with caution.
Initiation of prophylaxis
In Europe, it is common practice to begin anticoagulant prophylaxis 10-12 hours before surgery. In North America, the practice is to begin treatment 12-24 hours following surgery. The ninth edition of the ACCP guidelines suggested that for most major elective orthopedic surgical procedures, the first dose of LMWH may be administered either before or after surgery, [10] though meta-analyses suggest little advantage for preoperative initiation.
A study by Bergqvist and Hull seemed to suggest that starting a half-dose of anticoagulation 6 hours after surgery may deliver more effective prophylaxis without a significant increase in bleeding risk. [71] Patients with a high risk of bleeding should have the first postoperative dose delayed 12-24 hours after surgery. In a meta-analysis of 33 trials, Leonardi et al reported an approximately 3% rate of bleeding complications from DVT prophylaxis in which the bleeding was severe enough to require a change of care. [72]
For additional information, see Deep Venous Thrombosis, and Pulmonary Embolism.
Emerging Methods of Deep Venous Thrombosis Prophylaxis
An ideal anticoagulant should be easy to administer (preferably oral), should be effective and safe with a minimum of possible complications or adverse effects, have a rapid onset, have a therapeutic half-life, and require minimal or no monitoring. The action of the anticoagulant should be predictable with few drug or dietary interactions, and it should be reversible. The drug should also be inexpensive. Unfortunately, these criteria are often difficult to achieve. Several anticoagulant agents exist today, and each of them incorporates some of these characteristics, but no single agent combines all these attributes.
Current research in anticoagulants involves investigations into drugs that act on various phases of the coagulation cascade. For convenience, the authors can arbitrarily divide the process into three phases: the initiation phase, the propagation phase, and the thrombin activity phase.
Drugs under investigation that act in the initiation phase include tissue factor pathway inhibitors (TFPIs) and nematode anticoagulant peptide (NAPc2). These drugs act through inhibition of the factor VIIa/tissue factor complex.
A number of newer synthetic direct or indirect inhibitors of thrombin or factor Xa are being tested. These have similarities to the currently approved fondaparinux. Phase II dose-finding trials in which the metapentasaccharide idraparinux was administered subcutaneously (SC) once each week to prevent the development of secondary VTE have been completed. A second class of orally active direct factor Xa inhibitors, which includes razaxaban, is also undergoing clinical phase II trials. [73]
Ximelagatran, a direct thrombin inhibitor consisting of an oral prodrug of melagatran, is rapidly absorbed through the GI tract, where it is converted to its active form, melagatran. It does not require monitoring, as it has a rapid onset of action, a predictable dose-response, and a therapeutic half-life. Also, like the other direct thrombin inhibitors, it does not affect the aPTT or PT.
The results reported by Francis et al in the New England Journal of Medicine showed that ximelagatran and warfarin did not differ significantly with respect to the incidence of major or minor bleeding. [74] The report also determined that ximelagatran was significantly more effective in preventing DVT after TKR than was warfarin.
In the United States, four studies showed that postoperatively initiated ximelagatran (24 mg twice daily) had efficacy similar to that of enoxaparin or warfarin in the prevention of VTE in patients undergoing hip or knee replacement. Overall, the incidence of bleeding events and transfusion rates were not markedly different from those of comparator anticoagulants. Some patients experienced transient elevations of liver enzyme levels, which returned to normal after cessation of treatment. [75]
The FDA Cardiovascular and Renal Drug Advisory Committee (CRAC) reviewed the ximelagatran clinical program to propose the following three indications [76] :
-
Prevention of VTE in patients undergoing TKR
-
Prevention of stroke and other thromboembolic complications associated with atrial fibrillation
-
Secondary prevention of VTE after an episode of acute DVT
The committee reviewed data from 30,698 subjects and included five phase III studies, which led the CRAC to use the finding of ximelagatran hepatic toxicity as a key feature for an unfavorable benefit/risk ratio of ximelagatran for the three proposed indications. A report by Colwell and Mouret, however, indicated that melagatran/ximelagatran has been approved in the European Union for the prevention of VTE in patients undergoing elective hip or knee replacement surgery. [77] Boudes reported on the challenges and risk analysis to be learned from the ximelagatran FDA CRAC findings. [76]
Drugs that act on the third stage of the coagulation cascade, the thrombin activity phase, include the direct thrombin inhibitors. These drugs specifically inactivate thrombin and are independent of ATII). Included in this group are the hirudins and their derivatives made by recombinant DNA techniques.
Originally, hirudin was isolated from leech salivary gland tissue. The new drugs include bivalirudin and lepirudin. A randomized, multicenter, double-blind study of hirudin versus heparin in patients with THRs demonstrated that DVT and proximal DVT rates were decreased substantially in the hirudin group.
Another class of drugs acting at the third level of the coagulation cascade includes the noncovalent inhibitor argatroban, which is a carboxylic acid derivative that has been approved for use in the treatment of HIT.
In a randomized, controlled study of 90 patients undergoing total knee arthroplasty, Izumi et al found that intraoperative transcutaneous electrical nerve stimulation (TENS) had a significant effect with regard to prevention of DVT prophylaxis, preventing both venous stasis and blood hypercoagulability. [78]
For additional information, see Deep Venous Thrombosis, and Pulmonary Embolism.
Conclusion
Major surgical and high-risk orthopedic procedures place patients at risk for DVT and VTE, including PE. Complications of DVT include postphlebitic syndrome or death from PE. Therefore, prophylaxis with anticoagulant medications, as well as the adjunctive use of mechanical devices, is essential. The most effective treatment protocol for a patient must be determined on a case-by-case basis and account for the risk-benefit ratio in each situation. A risk stratification protocol, such as that developed by the ACCP, is recommended to determine the appropriate level and method of treatment.
Preventing VTE is always a tradeoff between the potential life-saving benefit of prophylaxis and the risk of hemorrhage. Therefore, even with adequate anticoagulant prophylaxis, DVT can and does develop. A study by Schiff et al revealed a 14% incidence of VTE following major orthopedic procedures, particularly TKR, in which standard prophylactic measures had been applied. [79] Continued vigilance and a high index of suspicion on the part of the medical staff is called for in this group of patients.
For fuller information on DVT and PE, please see Deep Venous Thrombosis and Pulmonary Embolism.
-
Comparison of binding sites for standard heparin and low-molecular-weight heparin.
-
Time course of deep venous thrombosis risk.