Practice Essentials
Cushing syndrome is caused by prolonged exposure to elevated levels of either endogenous or exogenous glucocorticoids. Exogenous use of glucocorticoids should always be considered and excluded in the etiology of Cushing syndrome. This article focuses on endogenous Cushing syndrome. Endogenous glucocorticoid overproduction, or hypercortisolism, can be dependent on or independent of adrenocorticotropic hormone (ACTH). [1, 2]
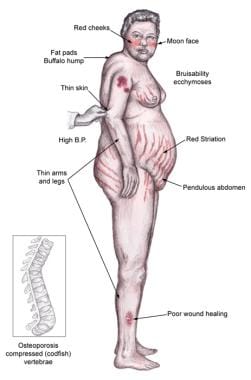
Individuals with Cushing syndrome can develop moon facies, facial plethora, supraclavicular fat pads, buffalo hump, truncal obesity, and purple striae, as shown in the image below.
Individuals often complain of proximal muscle weakness, easy bruising, weight gain, hirsutism, and, in children, growth retardation. Hypertension, osteopenia, diabetes mellitus, and impaired immune function may occur.
The form of Cushing syndrome known specifically as Cushing disease results when the pituitary gland overproduces ACTH due to a benign pituitary tumor.
Also see Glucocorticoid Therapy and Cushing Syndrome and Iatrogenic Cushing Syndrome.
Signs and symptoms of endogenous Cushing syndrome
Along with the general manifestations of Cushing syndrome, signs and symptoms specifically associated with the endogenous form include the following:
-
Patients with an adrenocorticotropic hormone (ACTH)–producing pituitary tumor - Headaches, polyuria, nocturia, visual problems, or galactorrhea
-
Patients with tumor mass effect on the anterior pituitary - Hyposomatotropism, hypothyroidism, hyperprolactinemia or hypoprolactinemia, hypogonadism
-
Patients with an adrenal carcinoma as the underlying cause of Cushing syndrome - Rapid onset of symptoms of glucocorticoid excess in conjunction with hyperandrogenism presenting as virilization in women or feminization in men
Workup in endogenous Cushing syndrome
Laboratory studies
Currently, four methods are accepted for the diagnosis of Cushing syndrome: the urinary free cortisol level, the low-dose dexamethasone suppression test, the evening serum and salivary cortisol level, and the dexamethasone–corticotropin-releasing hormone test.
In a patient in whom the diagnosis of Cushing syndrome has been established, an undetectable plasma ACTH with a simultaneously elevated serum cortisol level is diagnostic of ACTH-independent Cushing syndrome. ACTH-independent Cushing syndrome is most often due to a primary cortisol-producing adrenal adenoma or carcinoma, assuming exogenous glucocorticoid use has been excluded. A plasma ACTH (measured by an immunoradiometric assay) of less than 5 pg/mL is suggestive of a primary adrenal tumor. An ACTH level greater than 20 pg/mL (4.4 pmol/L) is consistent with ACTH-dependent Cushing syndrome. An ACTH level between 5 and 20 pg/mL is equivocal.
Imaging studies
Imaging studies for Cushing syndrome should be performed after the biochemical evaluation has been performed.
An abdominal computed tomography (CT) scan is recommended if a primary adrenal pathology is suspected.
If a pituitary source of excess ACTH is suspected, patients should undergo a contrast-enhanced magnetic resonance imaging (MRI) study of the pituitary.
Chest and abdominal CT scans should be performed in patients with suspected ectopic ACTH production.
Octreotide scintigraphy may be helpful in detecting ectopic ACTH tumors, because some neuroendocrine tumors typically have cell-surface receptors for somatostatin.
Procedures
Inferior petrosal sinus sampling (IPSS) is useful in distinguishing a pituitary source from an ectopic source of ACTH.
Management of endogenous Cushing syndrome
Pharmacotherapy
Medications used in the management of Cushing syndrome include the following:
-
11-beta-hydroxylase inhibitor - Osilodrostat
-
Somatostatin analogs - Pasireotide
-
Adrenal steroid inhibitors - Metyrapone, ketoconazole, etomidate
-
Glucocorticoid receptor antagonist - Mifepristone
-
Adrenolytic agents - Mitotane
Surgical therapy
The treatment of choice for endogenous Cushing syndrome is surgical resection of the causative tumor. The primary therapy for Cushing disease is transsphenoidal surgery, and the primary therapy for adrenal tumors is adrenalectomy.
Other surgical interventions include the following:
-
Bilateral adrenalectomy
-
Unilateral adrenalectomy
-
Resection of carcinomas
Pathophysiology
Endogenous glucocorticoid overproduction, or hypercortisolism, that is independent of ACTH is usually due to a primary adrenocortical neoplasm (most commonly an adenoma and rarely a carcinoma). Bilateral micronodular hyperplasia (primary pigmented nodular adrenocortical disease) and macronodular hyperplasia are rare causes of Cushing syndrome.
Ectopic cortisol secretion from a case of ovarian carcinoma has been reported as a cause of ACTH-independent Cushing syndrome. [3]
ACTH level in ACTH-independent Cushing syndrome is low due to the negative feedback to pituitary corticotroph cells from a high level of serum cortisol. ACTH-dependent Cushing syndrome is characterized by elevated ACTH levels. Elevated ACTH levels are usually due to an anterior pituitary tumor, which is classic Cushing disease (60-70%). Nonpituitary ectopic sources of ACTH, such as small-cell lung carcinoma (oat cell carcinoma), carcinoid tumor, medullary thyroid carcinoma, or other neuroendocrine tumors can result in high ACTH levels and sequentially hypercortisolism.
Ectopic corticotropin-releasing hormone (CRH) secretion leading to increased ACTH secretion is associated with a very rare group of cases of Cushing syndrome. [4]
A study by Tatsi et al indicated that in children with endogenous Cushing syndrome, the absolute lymphocyte count is abnormally low, while the total white blood cell (WBC) and absolute neutrophil counts are abnormally high, with a correlation found between these changes and the severity of Cushing syndrome. It was also found that successful treatment of the syndrome returns the WBC count to normal. [5]
Etiology
The following conditions may cause endogenous glucocorticoid overproduction.
ACTH-independent Cushing syndrome
See the list below:
-
Primary adrenal lesions
Overproduction of glucocorticoids may be due to an adrenal adenoma, adrenal carcinoma, or macronodular or micronodular adrenal hyperplasia. The zona fasciculata and zona reticularis layers of the adrenal cortex normally produce glucocorticoids and androgens. Glucocorticoid-secreting tumors are derived from these cells and, thus, may secrete both glucocorticoids and androgens.
In general, excess androgen secretion is suggestive of an adrenal carcinoma rather than an adrenal adenoma. These glucocorticoid-producing tumors do not usually secrete aldosterone, which is produced in the zona glomerulosa layer of the adrenal cortex.
The Carney complex is a familial form of micronodular hyperplasia of the adrenal gland. It is an autosomal dominant disorder and ACTH-independent cause of Cushing syndrome. Pigmented skin lesions and mesenchymal and endocrine tumors characterize this disorder. Cushing syndrome may be overt, subclinical, cyclical, or periodic.
Primary bilateral macronodular adrenal hyperplasia is uncommon and characterized by multiple nonpigmented nodules that are greater than 10 mm in diameter and enlarged adrenal glands. The exact etiology of this condition is not quite clear; however, genetic mutations, paracrine ACTH secretion, and aberrant hormone receptors have been reported to play a role in its pathogenesis.
McCune-Albright syndrome is a rare cause of precocious puberty. It is associated with hyperfunction of the adrenal glands that may lead to Cushing syndrome. [6]
-
Ectopic cortisol secretion from a case of ovarian carcinoma has been reported as a cause of ACTH-independent Cushing syndrome. [3]
ACTH-dependent Cushing syndrome
See the list below:
-
ACTH-producing pituitary adenoma
Pituitary adenomas that secrete ACTH are derived from corticotroph cells in the anterior pituitary.
ACTH secreted by corticotroph cells is released into the circulation and acts on the adrenal cortex to produce hyperplasia and stimulate the secretion of adrenal steroids.
These adenomas, if large, can result in loss of production of other anterior pituitary hormones (thyroid-stimulating hormone [TSH], follicle-stimulating hormone [FSH], luteinizing hormone [LH], growth hormone, and prolactin).
Nelson syndrome has been described as corticotroph tumor progression seen in patients who had bilateral adrenalectomy as radical treatment for Cushing disease. In a retrospective study looking at 53 Cushing disease patients who underwent bilateral adrenalectomy without pituitary irradiation, [7] corticotroph tumor progression was noted to be present in half of the patients, mostly within 3 years of surgery. Patients with a shorter duration of Cushing disease and a high plasma ACTH concentration in the year after adrenalectomy were more likely to develop Nelson syndrome. An enlarging corticotroph tumor can manifest clinically with compressive symptoms such as headache, vision change, and ocular palsy, and with hyperpigmentation due to very high ACTH concentrations.
-
Ectopic ACTH secretion is caused by small-cell lung tumors, carcinoid tumors, or other tumors with neuroendocrine origin. These tumors themselves can secrete ACTH, which subsequently stimulates the adrenal glands to make more cortisol.
-
Ectopic CRH secretion leading to increased ACTH secretion is associated with a very rare group of cases of Cushing syndrome. [4]
Epidemiology
Identifying the exact incidence of Cushing syndrome is challenging because patients can present with a wide spectrum from subclinical, mild, cyclic, to severe Cushing syndrome. Therefore, the true incidence may be underestimated. In a European population–based study, the annual incidence of endogenous Cushing syndrome was reported to be 1.2-1.7 per million per year (Cushing disease), 0.6 per million per year (adrenal adenoma), and 0.2 per million per year (adrenal carcinoma). [8]
The female-to-male incidence ratio is approximately 5:1 for Cushing syndrome due to an adrenal or pituitary tumor. Ectopic ACTH production is more frequent in men than in women because of the higher incidence of lung tumors in this population.
The peak incidence of Cushing syndrome due to either an adrenal or pituitary adenoma is in persons aged 25-40 years. Ectopic ACTH production due to lung cancer occurs later in life.
Prognosis
Mortality in patients with Cushing syndrome was reported to be twice that of persons without the disease. [9] A systematic review and meta-analysis indicated that Cushing disease patients with persistent disease after surgery have higher mortality than those with initial remission and Cushing syndrome due to a benign adrenal adenoma. [9]
A literature review by Limumpornpetch et al reported the standardized mortality ratio for persons with benign endogenous Cushing syndrome to be 3.0. [10]
Morbidity and mortality associated with Cushing syndrome are related primarily to the effects of excess glucocorticoids. Exposure to excess glucocorticoids results in multiple medical problems, including hypertension, cardiovascular disease, obesity, osteoporosis, fractures, impaired immune function, impaired wound healing, glucose intolerance, and psychosis. A large primary pituitary tumor may also cause panhypopituitarism and visual loss. Prognosis is favorable if surgery is curative. The rare adrenocortical carcinomas are associated with a 5-year survival rate of 30% or less. Catastrophic medical crises such as perforated viscera [11] and opportunistic fungal infections [12] can occur in glucocorticoid excess states. High levels of endogenous glucocorticoids may mask the abdominal symptoms associated with catastrophic abdominal events such as perforated bowel.
In the above-mentioned study by Limumpornpetch and colleagues, excess mortality from benign endogenous Cushing syndrome resulted mainly from atherosclerotic cardiovascular disease, which accounted for 27.4% of total deaths. Other causes of death included infection (12.7%), cerebrovascular disease (11.7%), malignancy (10.6%), thromboembolism (4.4%), gastrointestinal disease (3.2%), adrenal insufficiency (3.0%), suicide (2.2%), and surgery (1.5%). Another 15.5% of deaths were from undetermined causes. [10]
Lack of cortisol leading to an adrenal crisis may occur in patients with endogenous Cushing syndrome who have undergone resection of an ACTH-producing or cortisol-producing tumor or who are taking adrenal steroid inhibitors.
Other potential complications associated with Cushing syndrome include the following:
-
Increased susceptibility to infections
-
Risk for adrenal crisis
A study by Davi' et al found that in ectopic Cushing syndrome, prognosis is influenced by the type of neuroendocrine tumor causing it, as well as by grading, the presence of distant metastases, the severity of the hypercortisolism, the existence of hypokalemia, and the presence of diabetes mellitus. The investigators also determined that survival and surgical cure rates are better for bronchial carcinoids than for occult tumors and pancreatic neuroendocrine tumors. [13]
Patient Education
Patients should be educated about adrenal crisis.
Instruction on specific medication use is indicated.
-
Physical findings in Cushing syndrome.
-
Diagnosis of Cushing syndrome.
-
Etiology of Cushing syndrome.
-
High power H and E of a pituitary adenoma showing a monotonous proliferation of bland cells. Not all pituitary adenomas result in Cushing syndrome, only those that produce ACTH.
-
Intermediate power H and E of an adrenal adenoma showing a monotonous proliferation of bland cells. Not all adrenal adenomas or carcinomas result in Cushing's syndrome; only those that produce ACTH.