Practice Essentials
Persistent pulmonary hypertension of the newborn (PPHN) is defined as the failure of the normal circulatory transition that occurs after birth. It is a syndrome characterized by marked pulmonary hypertension that causes hypoxemia secondary to right-to-left shunting of blood at the foramen ovale and ductus arteriosus.
Signs and symptoms
PPHN is often associated with the following signs and symptoms of perinatal distress:
-
Asphyxia
-
Tachypnea, respiratory distress
-
Respiratory acidosis
-
Loud, single second heart sound (S2) or a harsh systolic murmur (secondary to tricuspid regurgitation)
-
Low Apgar scores
-
Meconium staining
-
Cyanosis; poor cardiac function and perfusion
-
Systemic hypotension
-
Symptoms of shock
Idiopathic persistent pulmonary hypertension of the newborn can present without signs of acute perinatal distress. Marked lability in oxygenation is frequently part of the clinical history.
See Presentation for more information.
Diagnosis
Suspect PPHN whenever the level of hypoxemia is out of proportion to the level of pulmonary disease. Clinically, PPHN is most often recognized in term or near-term neonates, but it can occur in premature neonates. Preductal and postductal oxygen saturation measurements via pulse oximetry will often show a 10% or higher gradient difference, which is dependent on the magnitude of right-to-left shunting at the foramen ovale (with preductal saturations being higher). It is important to note that these findings are not specific to PPHN and must be differentiated from structural heart disease
In contrast to adult primary pulmonary hypertension, the newborn syndrome is not defined by a specific pressure of the pulmonary circulation but rather an abnormally elevated pulmonary vascular resistance. The diagnosis is confirmed regardless of the pulmonary arterial pressure, as long as it is accompanied by a right-to-left shunt and absence of congenital heart disease. [1]
Echocardiography is considered the most reliable noninvasive test to establish the diagnosis, assess cardiac function, and exclude associated structural heart disease.
See Differential Diagnosis for more information
Workup
Laboratory testing
-
Arterial blood gas levels (through an indwelling line [eg, umbilical arterial catheter or preductal peripheral arterial line]): To assess the pH, partial pressure of carbon dioxide (PaCO2) and the partial pressure of oxygen (PaO2) which might be higher in the preductal arterial line.
-
Complete blood cell count with differential: To evaluate for high hematocrit level (polycythemia and hyperviscosity syndrome may lead to or exacerbate PPHN); to determine whether an underlying sepsis or pneumonia is present
-
Coagulation studies (eg, platelet count, prothrombin time, partial thromboplastin time, international normalized ratio): To assess for coagulopathy (increased disease severity)
-
Serum electrolytes (eg, calcium) and glucose levels
Imaging studies
-
Chest radiography: To assess for presence of underlying parenchymal lung disease (eg, meconium aspiration syndrome, pneumonia, surfactant deficiency) and/or to exclude underlying disorders (eg, congenital diaphragmatic hernia); see the image below
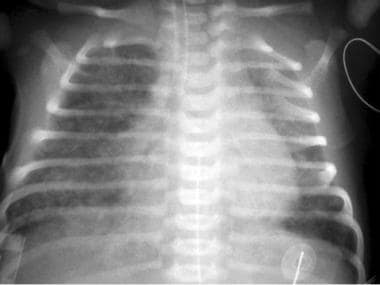 Persistent pulmonary hypertension of the newborn (PPHN). Meconium aspiration in a neonate. This radiograph was obtained shortly after birth and shows ill-defined, predominantly perihilar opacities in the lungs; these are more severe on the right than on the left. The lungs are hyperexpanded. The neonate's heart size is within normal limits.
Persistent pulmonary hypertension of the newborn (PPHN). Meconium aspiration in a neonate. This radiograph was obtained shortly after birth and shows ill-defined, predominantly perihilar opacities in the lungs; these are more severe on the right than on the left. The lungs are hyperexpanded. The neonate's heart size is within normal limits.
-
Echocardiography: To screen and assist in making the diagnosis of PPHN and to rule out a structural heart lesion
-
Echocardiography with Doppler and color-flow mapping: To assess presence/direction of the intracardiac shunt at the ductus arteriosus and foramen ovale, as well as estimate the pulmonary arterial systolic/diastolic pressures
-
Cranial ultrasonography: To assess for intraventricular bleeding and for peripheral areas of hemorrhage or infarct if ECMO is being considered
-
Cranial ultrasonography with Doppler flow: To assess whether a nonhemorrhagic infarct is present
-
Brain computed tomography scanning or magnetic resonance imaging: To evaluate for central nervous system injury
Procedures
-
Cardiac catheterization: Rarely utilized to exclude congenital heart disease (eg, obstructed anomalous pulmonary venous return, pulmonary vein stenosis) because echocardiographic findings are typically diagnostic
See Workup for more information.
Management
The treatment strategy for PPHN is aimed at maintaining adequate systemic blood pressure, decreasing pulmonary vascular resistance, ensuring oxygen release to tissues, and minimizing lesions induced by high levels of inspired oxygen and ventilator high pressure settings.
General management principles include the following:
-
Continuous monitoring of oxygenation, blood pressure, and perfusion
-
Maintaining a normal body temperature
-
Correction of electrolytes/glucose abnormalities and metabolic acidosis
-
Nutritional support
-
Minimal stimulation/handling of the newborn
-
Minimal use of invasive procedures (eg, suctioning)
Medical therapy
PPHN treatment may consist of the following:
-
Inotropic support (eg, dopamine [first line in the absence of cardiac dysfunction], dobutamine, milrinone)
-
Surfactant administration: For premature and full-term newborns with parenchymal lung disease
-
Endotracheal intubation and mechanical ventilation: To maintain normal functional residual capacity by recruiting areas of atelectasis; to avoid overexpansion
-
High-frequency ventilation: Used in newborns with underlying parenchymal lung disease and low lung volumes; therapy is best in centers with clinicians experienced in achieving/maintaining optimal lung distention
-
Correction of hypoglycemia, hypocalcemia, acidosis, and alkalosis
-
Induced paralysis: Controversial; paralytic agents are typically reserved for newborns who cannot be treated with sedatives alone (Note: paralysis, especially with pancuronium, may promote atelectasis of dependent lung regions and promote ventilation-perfusion mismatch.)
Pharmacotherapy
-
Inhaled pulmonary vasodilators (eg, nitric oxide) and supplemental oxygen
-
Systemic vasodilators are potentially beneficial for chronic PPHN after the newborn period (eg, prostacyclin, phosphodiesterase inhibitors, endothelin receptor antagonists)
-
Prostaglandin E1 if the ductus arteriosus is closed or restrictive in the setting of suprasystemic pulmonary artery pressures and/or right ventricular dysfunction leading to poor systemic perfusion
See Treatment for more information.
Background
Persistent pulmonary hypertension of the newborn (PPHN) is defined as the failure of the normal circulatory transition that occurs after birth. It is a syndrome characterized by marked pulmonary hypertension that causes hypoxemia secondary to right-to-left extrapulmonary shunting of deoxygenated blood. Clinically, PPHN is most often recognized in term or near-term neonates, but it is being increasingly recognized in preterm infants as well. [4]
Because virtually all newborns are born with elevated pulmonary pressures and have a patent foramen ovale and a patent ductus arteriosus immediately after birth, the presence of elevated pulmonary vascular resistance beyond baseline may lead to a right-to-left shunting of blood and severe hypoxemia. With inadequate pulmonary perfusion, neonates are at risk for developing refractory hypoxemia, hypercarbia, and acidosis.
The diagnosis of PPHN is confirmed by echocardiography. The cardinal findings include abnormal right ventricular dilatation, leftward deviation of the interventricular septum, tricuspid regurgitation, and right-to-left shunting at the levels of the patent foramen ovale and patent ductus arteriosus. [5]
Fetal pulmonary hypertension
Pulmonary hypertension is a normal and necessary state for the fetus, because the placenta, not the lungs, serves as the organ of gas exchange. Most of the right ventricular output crosses the ductus arteriosus to the aorta, and only 13-21% of the combined ventricular output is directed to the pulmonary vascular bed. [4]
Mechanisms that maintain high pulmonary vascular resistance in utero include low fetal oxygen content, fluid-filled alveoli causing compression of the pulmonary blood vessels, and the presence of vasoconstrictor mediators, such as endothelin-1, thromboxane, and leukotriene.
Normal cardiopulmonary transition
A dramatic cardiopulmonary transition occurs at birth, as pulmonary blood flow increases 8- to 10-fold and pulmonary arterial pressure decreases by 50% within 24 hours. This is due to a marked increase in oxygen tension, the establishment of an air-liquid interface, and rhythmic distention of the lungs from breathing. [6, 7] The most critical signals for these transitional changes are mechanical distention of the lungs, a decrease in carbon dioxide tension, and an increase in oxygen tension in the lungs. Endothelial nitric oxide (NO) production in the lungs increases after birth as a result of the increased blood flow and oxygenation. The NO then mediates pulmonary vasodilation via cyclic guanosine monophosphate (cGMP). Cyclic adenosine monophosphate (cAMP) is increased after birth by the arachidonic acid-prostacyclin pathway which promotes smooth muscle cell relaxation.
Failure of circulatory transition
In some newborns, this normal decrease in pulmonary vascular tone does not occur, resulting in PPHN. This results in shunting of blood away from the lungs and severe central hypoxemia.
Severe PPHN can be associated with poor cardiac output and shock, signs of which include tachycardia, ashen or gray color, capillary refill time more than 3 seconds, oliguria, hypotension, and lactic acidosis. This is commonly seen when the ductus arteriosus is restrictive and right-to-left shunting is compromised at this level or at the foramen ovale.
Neurologic sequelae
Although most surviving newborns with PPHN have normal neurodevelopmental outcomes, as many as 25% have significant neurodevelopmental sequelae with a high prevalence of both expressive and receptive linguistic deficits. [8]
Etiology
Pulmonary endothelium-derived vasodilators
Several events take place after birth as a fetus transitions from placental gas exchange to that taken care of by the lungs. At birth, the umbilical cord is clamped, which removes the low-resistance placenta circulation and increases the systemic circulation. In addition, pulmonary blood pressure begins to rapidly fall, leading to an increase in pulmonary blood flow.
The drop in pulmonary vascular resistance is due to several factors, including ventilation of the lungs causing an increase in oxygen tension and the release of several vasoactive factors by the pulmonary endothelium, including endothelin-1 (ET-1), nitric oxide (NO), and prostacyclin (PGI2). Endothelial nitric oxide synthase (or nitric oxide synthase type 3) is the most extensively studied enzyme in persistent pulmonary hypertension of the newborn (PPHN). When activated by shear stress or adenosine triphosphate, it converts L-arginine into NO and L-citrulline.
NO is a potent vasodilator and its production and release by the pulmonary endothelium rapidly increases after birth. The increase in oxygen tension is an important stimulator for this process. NO stimulates the soluble guanylate cyclase enzyme in the pulmonary vascular smooth muscle cells, leading to the conversion of guanosine triphosphate nucleotide into cyclic guanosine monophosphate (cGMP). The increase in intracellular cGMP leads to a decrease in calcium influx and relaxation of smooth muscle cells by stimulating protein kinase G. [9, 10] cGMP is down-regulated by phosphodiesterase 5 activity. Phosphodiesterase 5, which is abundantly expressed in lung tissue, particularly during fetal life, is a key regulator of perinatal pulmonary circulation. [11]
Experimental studies of chronic pulmonary hypertension in newborn animals have demonstrated impaired endothelial release of NO and increased production of vasoconstrictors (eg, endothelin-1). [12] Endothelin-1, a 21–amino acid polypeptide elaborated by the endothelium, is a vasoconstrictor to the pulmonary arteries and enhances oxygen formation that depletes NO bioavailability and promotes the growth of the pulmonary artery muscular layer.
Vascular endothelial growth factor (VEGF) is another potent endothelial cell mitogen and regulator of angiogenesis. In vivo, inhibition of VEGF receptors in normal fetal sheep results in impaired vascular growth and leads to pulmonary hypertension. [13]
Genetic factors may increase susceptibility to pulmonary hypertension. Strong links between PPHN and polymorphisms of the carbamoyl phosphate synthase gene have been reported. [14] However, the importance of this finding is uncertain, and further work is needed in this area. More recently, investigators have described an association between polymorphisms in urea cycle enzyme genes and PPHN; they reported three single-nucleotide polymorphisms (SNPs) (rs41272673, rs4399666, and rs2287599) in the carbamoyl phosphate synthase 1 gene (CPS1) were significantly associated with PPHN. [15] In addition, a recognized emerging cause of pediatric pulmonary hypertension is rare variants in the T-box transcription gene (TBX4); however, their pathophysiology and how they contribute to PPHN are unclear. [16] CPS1, NOTCH3, and SMAD9 appear to be risk genes for late preterm and term PPHN. [17] Larger studies are needed to replicate these findings.
Selective serotonin reuptake inhibitors (SSRIs), commonly prescribed antidepressants, have been reported to be associated with PPHN, especially during the third trimester of pregnancy. The prevalence of PPHN in newborns exposed to SSRIs in the second half of pregnancy is increased 6-fold. However, one recent study involving 1104 infants born to mothers who received antidepressants in the third trimester and an equal number of controls failed to demonstrate this association. [18]
Therefore, the US Food and Drug Administration (FDA) has stated that it is premature to reach any conclusion about a possible link between SSRI use in pregnancy and PPHN. They are advising healthcare professionals not to alter their current clinical practice of treating depression during pregnancy and to report any adverse events to the FDA MedWatch program. [19]
PPHN is most commonly associated with one of the following underlying etiologies [20] :
-
Acute pulmonary vasoconstriction
-
Hypoplasia of the pulmonary vascular bed (commonly seen with congenital diaphragmatic hernia)
-
Idiopathic pulmonary hypertension
There also appears to be a complex relationship between PPHN and cesarean delivery, including the following factors [21] :
-
Iatrogenic prematurity
-
Higher rates of late preterm delivery
-
Lack of physiologic changes of labor
-
Limited pulmonary vasodilator synthesis
-
Lower levels of protective antioxidants
-
Greater risk of respiratory distress syndrome, with a concomitant rise in endothelin-1 levels
Acute pulmonary vasoconstriction
The most commonly encountered scenario in PPHN is acute pulmonary vasoconstriction due to acute perinatal events, such as:
-
Alveolar hypoxia secondary to parenchymal lung disease, such as meconium aspiration syndrome, respiratory distress syndrome, or pneumonia
-
Hypoventilation resulting from asphyxia or other neurologic conditions
-
Hypothermia
-
Hypoglycemia (defined as < 40 mg/dL in neonates per the American Association of Pediatrics)
Hypoplasia of the pulmonary vascular bed
Hypoplasia of the pulmonary vascular bed is another cause of persistent pulmonary hypertension of the newborn.
Congenital diaphragmatic hernia is an abnormality of diaphragmatic development that allows the abdominal viscera to enter the chest and compress the lung, impairing growth.
Oligohydramnios may also produce pulmonary hypoplasia and associated persistent pulmonary hypertension of the newborn.
A congenital cystic adenomatoid malformation may lead to lung hypoplasia, but PPHN is not a common finding in this condition. [22]
Idiopathic pulmonary hypertension
Idiopathic pulmonary hypertension accounts for approximately 10% of the cases of PPHN. It is caused by impaired pulmonary relaxation after birth in the absence of parenchymal lung disease. One cause of idiopathic PPHN is constriction, or premature closure of the ductus arteriosus in utero, which can occur after exposure to aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, ibuprofen, naproxen) during the third trimester. Evaluation of infants at autopsy shows significant remodeling of their pulmonary vasculature, with vascular wall thickening and smooth muscle hyperplasia. [23] Furthermore, the smooth muscle extends to the level of the intra-acinar arteries, which does not normally occur until late in the postnatal period. As a result, infants do not vasodilate their pulmonary vessels adequately in response to birth-related stimuli, and they present with hypoxemia and hyperlucent lung fields on radiography, which is termed black lung PPHN.
Epidemiology
Incidence in the United States
The incidence of persistent pulmonary hypertension in the newborn (PPHN) in the United States has been reported to range from 0.4 to 6.8 per 1000 live births. [4] Although PPHN has been traditionally thought of as a diagnosis in term newborns, it is increasingly being recognized in preterm infants. One study reported an incidence of 5.4 per 1000 live births in infants 34-36 weeks' gestation. [24]
An analysis of the North American Pediatric Pulmonary Hypertension Network Registry (2014-2018) found "significant racial variability in the prevalence of pulmonary hypertension subtypes and survival outcomes among children with pulmonary hypertension." [25] For example, Black neonates had a higher prevalence of PPHN and an increased mortality risk, whereas White neonates had a greater prevalence of congenital diaphragmatic hernia. [25]
Prognosis
Morbidity and mortality
Relatively recent advancements in the treatment and management of PPHN (such as inhaled nitric oxide and extracorporeal membrane oxygenation) have helped to reduce the morbidity and mortality of this disease; however, the mortality rate still remains approximately 10% in those infants with moderate to severe disease, and it is higher in infants with other morbidities such as pulmonary hypoplasia and congenital diaphragmatic hernia. [26] PPHN has been associated with significant long-term morbidities in up to 25% of infants, including neurodevelopmental impairments and hearing difficulties. [26]
A 2019 report of a retrospective California population-based study (2005-2012) found a large postdischarge morbidity burden of infants with PPHN in their first year of life, including those with mild PPHN and causes involving pulmonary vascular changes that have been believed to be of short duration and recoverable. [27] The investigators also identified the following as risk factors for postdischarge mortality and morbidity in the first year of life of newborns with PPHN [27] :
-
Hispanic ethnicity
-
Small for gestational age
-
Severe PPHN
-
PPHN etiology (eg, congenital diaphragmatic hernia, meconium aspiration syndrome)
-
Persistent pulmonary hypertension of the newborn (PPHN). Meconium aspiration in a neonate. This radiograph was obtained shortly after birth and shows ill-defined, predominantly perihilar opacities in the lungs; these are more severe on the right than on the left. The lungs are hyperexpanded. The neonate's heart size is within normal limits.


