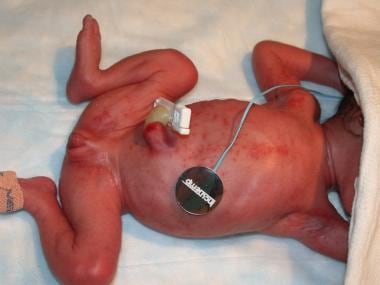
Overview
The risk for invasive fungal infections is high in very low birth weight (VLBW) infants (< 1500 g) and highest for infants born at the youngest gestational ages who survive past the immediate postnatal period. [1, 2] These immunocompromised infants usually require invasive therapies, such as central vascular catheters and endotracheal tubes, and they are exposed to broad-spectrum antibiotics and parenteral nutrition. In addition, these infants occasionally receive postnatal steroids and gastric acid inhibitors. All of these factors place them at high risk for fungal infection. Prior to studies of and broad institution of antifungal prophylaxis in high-risk preterm infants, the incidence of fungal infections had been rising in infants born at less than 1000 g, with the ensuing resuscitation and survival of more and more infants. [3, 4]
Most fungal infections in preterm neonates are due to Candida species (spp); a much smaller number of infections may be attributed Malassezia, Zygomycetes, or Aspergillus pathogens. Candida spp are commensal organisms that colonize the skin and mucosal surfaces, and they adhere to catheter surfaces. In centers that use antifungal prophylaxis, Candida infections are few and due to non-albicans species. Whereas, in centers that do not use antifungal prophylaxis, C albicans and C parapsilosis account for the majority of infections, with one other common non-albicans species as well (eg, C glabrata or C tropicalis). Candida can invade the bloodstream of preterm infants because of their immature and easily injured skin and mucosal membrane barrier defenses, and the fungus can disseminate because of the infants' immature immune systems. For these reasons, fungal infections are often difficult to eradicate in the preterm infant and, in cases of candidemia, central venous catheter removal is critical for clearance and survival.
Although these immunocompromised infants are at increased risk during most of their hospital stay, they are at the highest risk of acquiring invasive fungal infections during the first weeks of life, when the most invasive therapies are performed and remain in place. Although an index of suspicion must always remain high, infection control, prophylaxis, and aggressive treatment (antifungal therapy and central catheter removal) during this period have the greatest potential to improve the outcome of this population.
Pathogenesis
The pathogenesis of fungal infections in preterm infants involves adherence, colonization, and dissemination (as shown in the image below).
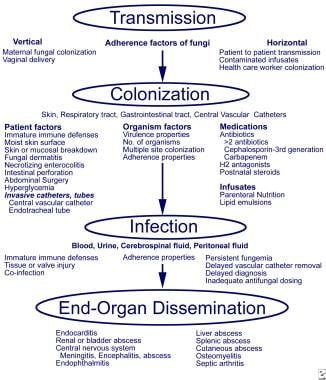 Fungal Infections in Preterm Infants. Pathogenesis and invasive fungal infections in very low birth weight infants. From Kaufman and Fairchild 2004, with permission.
Fungal Infections in Preterm Infants. Pathogenesis and invasive fungal infections in very low birth weight infants. From Kaufman and Fairchild 2004, with permission.
Adherence and the slow-growing nature of Candida facilitate its ability to colonize and disseminate into the bloodstream and body tissues before clinical signs and symptoms of infection become apparent. Surface glycoproteins play a role in fungal adherence. One such surface adherence glycoprotein is INT1p, which binds to beta-integrins present on the endothelium and white blood cells (WBCs). The absence of a functional INT1 gene diminishes adherence in yeast cells but not filamentous forms.
The preterm infant is immunocompromised and frequently exposed to broad-spectrum antibacterial medications. Investigators have studied the effect of steroids and antibiotics in mice orally inoculated with C albicans to mimic conditions in the preterm infant. [5] Antibiotic treatment alone led to increased Candida colonization but did not affect dissemination. When dexamethasone as immunosuppression was added to the antibiotic regimen to more closely mimic the preterm infant's immunocompromised state, both colonization and dissemination increased in these animal models. Dexamethasone plus antibiotics led to an increase in the percentage of filamentous forms in the gastrointestinal (GI) tract compared with antibiotics alone. In addition, introduction of C albicans strains with two functional copies of the INT1 gene increased the number of fungi colonizing the cecum and disseminating to extraintestinal sites. [5]
C albicans is dimorphic, having both yeast and filamentous forms (eg, hyphae, pseudohyphae, germ tubes), and is assumed to have increased virulence in immunocompromised patients because of the filamentous forms. Filamentous forms contribute to the severity of infection (eg, C albicans is associated with the highest mortality), although species that do not form filaments, such as C glabrata, can colonize and cause invasive disease in very low birth weight (VLBW) infants.
To further examine the role of yeast and filamentous forms, researchers intravenously or orally infected antibiotic-treated and dexamethasone-treated mice using three strains of C albicans: (1) a wild-type strain with both yeast cell and filamentous forms, (2) a strain with only yeast cells, and (3) a strain that was constitutively filamentous. [6] Mortality was significantly greater in both the wild-type (92%) and yeast-cell (56%) strains compared with the filamentous strain alone (0%). The filamentous strain had no dissemination, and cecal colonization was significantly less than that of the other two strains. The wild-type strain had diffuse hyphal invasion with increased tissue necrosis compared with the yeast-cell strain. The researchers speculated that the yeast forms are critically important for adherence and tissue dissemination, and that hyphal formation in the tissues contributes to parenchymal destruction. [6]
In preterm infants, both vertical and horizontal transmission leads to colonization of the skin, mucosal membranes (GI and respiratory tracts), and central vascular catheters (see the image below).
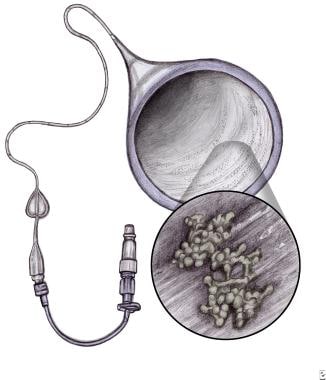 Fungal Infections in Preterm Infants. Percutaneous intravenous central catheter and fungal biofilm formation.
Fungal Infections in Preterm Infants. Percutaneous intravenous central catheter and fungal biofilm formation.
After exposure, patient factors, such as degree of prematurity, skin condition, endotracheal intubation, central vascular access, diseases (eg, necrotizing enterocolitis [NEC], focal bowel perforation [FBP]), and abdominal surgery, can contribute to fungal infection. Fungal factors that contribute to infection include the size of the inoculum and factors that favor colonization and proliferation (eg, use of broad-spectrum antibiotics, postnatal steroids, histamine type-2 [H2] antagonists, parenteral nutrition, or lipid emulsions [Malassezia spp]).
Invasive infection of the blood, urine, cerebrospinal fluid (CSF), or peritoneal fluid can lead to disseminated infection, which most commonly involves the heart, kidneys, central nervous system, eyes, and/or liver.
Risk Factors
Invasive fungal infection risk factors in the preterm infant are shown in the image below.
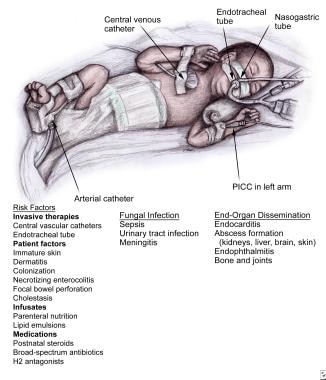 Fungal Infections in Preterm Infants. Risk factors for candidemia. PICC = peripherally inserted central catheter.
Fungal Infections in Preterm Infants. Risk factors for candidemia. PICC = peripherally inserted central catheter.
In the very low birth weight (VLBW) infant, colonization of the skin, mucosal membranes, and/or vascular catheters commonly precedes infection. Biofilm formation on catheters inhibits the host's defense mechanisms and the penetration of antifungal agents. Infusates may also become contaminated and directly seed the bloodstream.
Risk factors for Candida colonization and sepsis are similar. [7, 8] Central vascular catheters, vaginal delivery, use of third-generation cephalosporins, and high acuity are risk factors for C albicans infection. H2 antagonists, third-generation cephalosporins, central vascular catheters, parenteral nutrition and lipid emulsions, and high acuity are risk factors for C parapsilosis infection. Gastrointestinal (GI) disease (eg, necrotizing enterocolitis [NEC], focal bowel perforation [FBP]), exposure to fluconazole or antibiotics, prolonged hospitalization, and infection with other fungi increase the risk of sepsis due to C glabrata. GI mucosal injury, antibiotic suppression of bacterial flora, neutropenia, and parenteral nutrition increase risk of sepsis due to C tropicalis.
Patient risk factors and odds ratios (ORs) summarized from two multicenter studies are as follows [7, 9] :
-
Gestational age: For patients born at less than 25 weeks' gestation, the OR was 4.2. For patients born at less than 25-27 weeks' gestation, the OR was 2. For patients born at less than 32 weeks' gestation, the OR was 4.
-
Antibiotics: For patients who received third-generation cephalosporin or carbapenem treatment within 7 days prior to infection, the OR was 1.8. For patients who received two or more antibiotics prior to infection, the OR was 3.8.
-
Invasive therapies: For patients who received mechanical ventilation therapy, the OR was 10.7. For patients with a central venous catheter, the OR was 3.9.
-
Intravenous nutrition: For patients who received parenteral nutrition for longer than 5 days, the OR was 2.9. For patients who received lipid emulsion longer than 7 days, the OR was 2.9.
-
Medications: For patients using H2 antagonists, the OR was 2.4.
Diseases that increase risk for fungal sepsis are as follows:
-
Prior bloodstream infection: Patients with prior bloodstream infection may be more susceptible to infections and/or the effect of antibiotics on skin and mucosal microflora (OR, 8.02). [10]
-
FBP: If the GI tract is colonized with Candida spp, Candida peritonitis and sepsis can complicate bowel perforation in affected infants. [13]
-
Other GI diseases: Complicated GI disease in which infants receive nothing by mouth (not enterally fed) and/or antibiotics for longer than 7 days raises the risk for fungal sepsis. Examples include gastroschisis, omphalocele, intestinal atresias, tracheoesophageal fistula, and Hirschsprung disease. Complicated GI disease increases risk in both preterm and term infants (OR, 4.57). [10]
-
Candida dermatitis: As discussed above, skin inflammation caused by Candida spp may precede invasion into the bloodstream due to immature skin and host defenses, skin injury, large fungal burden, and C albicans virulence.
Candidal Infections
Candida species
Any Candida species may cause disease in neonates.
C albicans remains the most frequently isolated yeast species in infected neonates, followed by C parapsilosis infections, in centers not using antifungal prophylaxis. [14, 15] Because fluconazole prophylaxis is highly effective in preventing C albicans colonization and infection, those neonatal intensive care units (NICUs) that implement such prophylaxis have few infections and they are often non-albicans spp. Less common Candida spp include C glabrata and C tropicalis, and a small percentage of infections are due to C lusitaniae, C guilliermondii, or C dubliniensis.
Candidal infections
Very low birth weight (VLBW) infants with congenital Candida infection are more likely to present with severe infection, such as pneumonia, and widespread dermatitis with focal areas of superficial erosion and desquamation. [16, 17, 18]
Congenital cutaneous candidiasis: [19]
-
Skin findings are commonly present on the day of birth, appearing most frequently as a desquamating, maculopapular, papulopustular, and/or erythematous diffuse rash. Rash may present as well within a few days after birth. The skin involvement covers one or more of the following areas: face/scalp, chest, abdomen, perineal area, one or more extremity, and/or back.
-
The diagnosis can be made with a diffuse rash involving the body, extremities, face or scalp, and/or funisitis, presenting in the first week (≤7 days), with identification of Candida spp from skin or mucous membrane cultures, and/or by culture or staining of the placenta or umbilical cord. [19] Aerobic skin culture (and not only a fungal skin culture) should be obtained, as the differential diagnosis often includes gram-positive skin infections.
-
Congenital cutaneous candidiasis is invasive. Dissemination in preterm and term infants should be treated with systemic antifungal agents for a minimum of 14 days. Topical antifungal treatment is ineffective and associated with poor outcomes. Prompt systemic treatment at the time of rash presentation is critical for the prevention of dissemination and elimating mortality. Dissemination (~10%) and mortality (4%) can also occur even in term infants. [20] For affected term infants, discussing the need for evaluation of the infant's immune system for a primary immunodeficiency may be indicated.
-
Without prompt systemic treatment for congenital cutaneous candidiasis, infants are at greater risk of developing dissemination to the bloodstream: 66% in extremely low birth weight (ELBW) infants, 33% in those whose birth weight are 1000-2500 g, and 11% in term infants. For this reason, all affected infants should be treated with systemic antifungal therapy.
Congenital cutaneous candidiasis
An example of congenital cutaneous candidiasis is shown in the image below.
A diffuse, burnlike, erythematous, macular dermatitis with skin exfoliation is more likely to manifest with blood, urine, or cerebrospinal fluid (CSF) involvement than with a papulopustular rash in patients of any gestational age. [20]
Mucocutaneous candidiasis or Candida dermatitis
Mucocutaneous candidiasis or Candida dermatitis manifests postnatally with an erythematous papulopustular rash similar to congenital cutaneous candidiasis. As many as 70% of ELBW infants with candidal dermatitis develop bloodstream dissemination. [18, 21, 22] Preterm infants weighing less than 1500 grams with mucocutaneous candidiasis in the first weeks of life should be treated with systemic antifungal therapy for a minimum of 14 days. [16, 20]
Bloodstream infection (BSI)
Bloodstream infection with fungal species demonstrates clinical signs and symptoms similar to that of bacterial sepsis. The incidence of candidemia is reported as 2-6.8% among VLBW infants not receiving prophylaxis. [7, 14, 16, 23, 24, 25, 26, 27, 28] The incidence is higher in ELBW infants, ranging from 4% to 16%. [8, 14, 29, 30, 31] The incidence increases in an inverse linear pattern, from around 3% at 28 weeks' gestation to 24% at 23 weeks' gestation. [30, 31] Most importantly, candidemia can disseminate. Evaluation of the cardiac, renal, ophthalmologic, and central nervous systems is warranted.
Urinary tract infection (UTI)
UTIs are extremely common. Evaluation of late-onset sepsis should include a urine culture obtained via sterile bladder catheterization. Urinalysis is not helpful due to delay in pyuria and poor leukocyte esterase production. If the urine culture is positive for fungus, renal ultrasonography should be performed to detect fungus in the collecting system. [32] Candiduria develops in approximately 2.4% of VLBW infants and up to 6% of ELBW infants. [1] Studies have demonstrated similar mortality in infants with Candida UTI alone (26%) relative to Candida BSIs (28%) in ELBWs. [33, 34] These findings emphasize the need for prompt treatment for a minimum duration of 14 days in preterm infants.
Meningitis
The reported frequency of fungal meningitis among VLBW infants is 1.6%. [31, 35] The true incidence is likely higher, because lumbar punctures are not obtained in many VLBW infants at the onset of sepsis. Cell counts in preterm infants may not always be helpful, because the results may not be abnormal in the presence of meningitis.
Disseminated infection
Patients with disseminated infection may present with several entities. [11, 36, 37] These infections require a longer duration of treatment of 4-6 weeks or longer until resolution. Consultation with pediatric infectious disease specialists should be considered to help guide treatment decisions.
-
Endocarditis has been reported in 5-15% of candidemia cases.
-
Renal abscess is detected in 5% of patients with candidemia. It may occur in about 33% of VLBW infants with fungal UTIs.
-
Endophthalmitis: The literature reports an incidence of endophthalmitis in 3-6% of neonates with candidemia. Endophthalmitis occurs as multiple or single, yellow-white, raised lesions with indistinct (fluffy) or circular edges located in the posterior fundus or vitreous. It may affect one or both eyes. [39] Possibly due to earlier treatment, appropriate dosing, and prophylaxis, the incidence has been less 1% in relatively recent years.
-
Liver abscess occurs in 3% of preterm infants with candidemia. The likelihood is higher if there is a history of malpositioned umbilical venous catheter (UVC) in the liver. Liver ultrasonography should be performed if there is a history of malpositioned UVC, hepatomegaly, or significant change in liver enzymes results, and for patients with persistent candidemia (>5 days). [11, 12] Hepatic candidiasis is shown in the image below.
-
Splenic abscess: Splenic ultrasonography is recommended in patients with candidemia and should be performed if splenomegaly occurs or candidemia persists longer than 5 days.
-
Cutaneous abscess should be cultured and drained, if indicated.
-
Osteomyelitis: Evaluation for osteomyelitis should be considered in infants with infection who are not moving, have limited range of motion, or have swelling of an extremity.
-
Septic arthritis manifests with joint swelling. It may also occur several months after antifungal treatment. [40]
-
Peritonitis may occur with any bowel perforation, focal bowel perforation (FBP), and necrotizing enterocolitis (NEC). It can be a complication of any abdominal surgery.
Clinical Presentation and Differential Diagnosis
Clinical presentation
Although the very low birth weight (VLBW) infant with candidiasis can present with many of the nonspecific signs and symptoms associated with invasive bacterial infection, candidiasis signs/symptoms are often more subtle and indolent. Cultures should be obtained whenever sepsis is suspected. Cultures should be repeated after the initial evaluation if the infant does not clinically improve within 48 hours or if the infant's condition worsens. New-onset thrombocytopenia (< 100 × 109/L [< 100 X 103/µL, or < 100,000/µL]) is present in most cases of fungal sepsis, and decrease of an additional 50% to a mean platelet count below 50 X 109/L (< 100 X 103/µL, or < 100,000/µL). [41] Persistent thrombocytopenia below 50,000 × 109/L after 7 days of antifungal therapy may indicate end-organ dissemination with abscess.
Signs and symptoms in VLBW infants with candidemia are summarized according to incidence, as follows: [42, 43]
-
New-onset thrombocytopenia with platelet count below 100 × 109/L (< 100 X 103/µL, or < 100,000/µL): 84%
-
Decrease in platelet count by 50%
-
Immature-to-total neutrophil ratio of 0.2 or higher: 77%
-
Increase in apnea and/or bradycardia: 63%
-
Increase in oxygen requirement: 56%
-
Increase in assisted ventilation: 52%
-
Lethargy and/or hypotonia: 39%
-
Gastrointestinal symptoms (eg, gastric aspirates, distention, bloody stools): 30%
-
Hypotension: 15%
-
Glucose concentration above 140 mg/dL: 13%
-
White blood cell (WBC) count over 20 × 109/L (>20 X 103/µL, or >20,000/µL): 12%
-
Metabolic acidosis: 11%
-
Absolute neutrophil count (ANC) below 1.5 × 109/L (< 1500/µL): 3%
Differential diagnosis
The differential diagnosis for candidiasis includes the following conditions:
-
Gram-positive or gram-negative sepsis
-
Necrotizing enterocolitis (NEC)
-
Focal bowel perforation (FBP)
-
Abscess
-
Intracranial hemorrhage
-
Central vascular catheter thrombosis
Aspergillosis, Mucormycosis (Zygomycosis) and Malassezia Infections
Aspergillosis and mucormycosis are caused by filamentous fungi or molds; while uncommon, these infections occur and cause severe cutaneous infections in extremely preterm infants. [44, 45, 46] A comparison of neonatal fungal cutaneous manifestations of candidiasis, [19, 47] aspergillosis, [45, 46] and mucormycosis [44, 48] in preterm infants is outlined in the table below.
Cutaneous neonatal infections can occur due to the immature skin as well as preterm infants' underdeveloped macrophage chemotaxis and phagocytosis, which are the major host defenses against aspergillosis. Aspergillus presents in neonates most commonly as a cutaneous infection and rarely as pulmonary or disseminated disease. Voriconazole or echinocandins are the optimal first-line agents indicated in neonatal aspergillosis cases.
Environmental contamination of dust from hospital construction or faulty ventilation can carry Aspergillus spores that may settle in wounds or be inhaled. Regular cleaning of the ventilation systems in the neonatal intensive care unit (NICU) can prevent buildup of spores and appropriate containment of dust (often in the ceilings) during hospital renovation and construction.
Cutaneous mucormycosis presents as a black eschar at the site of local trauma or under a covered area or the back. [44] Other sites may include an intravenous (IV) catheter infiltrate or necrotizing enterocolitis (NEC). These spores are in the environment, most commonly in the soil and decaying organic matter, but they can be present in the air as well. Extreme prematurity and neutropenia are major patient risk factors for mucormycosis in the NICU. Mucor infections can often evolve into necrotizing soft-tissue infections. Adhesive tape, monitor leads, and wooden tongue blades used for splints in the NICU can become contaminated. Early diagnosis, administration of amphotericin B, and surgical debridement are necessary to prevent ulceration, necrosis, and rapid fatal dissemination. A high degree of suspicion is required; fungal culture or tissue biopsy can diagnose these right-angle, branched, nonseptated hyphae. Mortality may be as high as 61% from infections in neonates without prompt diagnosis and treatment. [28]
Table 1. Cutaneous Manifestations of Neonatal Fungal Infections (Open Table in a new window)
Candidiasis (Candida spp) |
Aspergillosis (Aspergillus fumigatus) |
Mucormycosis (Rhizopus and Mucor spp) |
|
|---|---|---|---|
| Dermatologic Findings |
|
|
|
| Evaluation | Culture (aerobic or fungal) | Culture (aerobic or fungal) or biopsy | Fungal culture or biopsy |
| Diagnosis |
|
Dichotomously branched and septate hyphae, identified by microscopic examination of 10% KOH wet prep or Gomori methenamine-silver nitrate stain |
Broad nonseptate or, rarely, pauciseptate (few septa) hyphae that become twisted and ribbonlike and branch at right angles from the parent hyphae. Pauciseptate differentiates from aspergillosis. |
Treatment: Start empirically when rash/lesions are present |
Amphotericin B and other susceptible antifungals | Voriconazole, echinocandins | Amphotericin B and surgical debridement, if indicated |
Malassezia infections
Presentation of infection with Malassezia organisms is similar to that seen with invasive candidiasis. Infection does not routinely disseminate; therefore, end-organ surveillance is needed only if Malassezia spp are persistently isolated from several cultures.
M furfur is a lipid-dependent fungus that may colonize central venous catheters when lipid emulsions are infused. It can also colonize the skin and gastrointestinal (GI) tract. Horizontal transmission is common. These fungi readily grow in Sabouraud medium coated with sterile olive oil. Treatment can include any one of the following measures:
-
Stopping lipid infusions for 48-72 hours
-
Stopping lipid infusions for 48-72 hours and administering amphotericin B for 7 days
-
Stopping lipid infusions for 48-72 hours and removing the central venous catheter
-
Removing the central venous catheter
M pachydermatis is not an obligate lipophilic organism. It has been reported to cause sepsis, urinary tract infection, and meningitis in very low birth weight (VLBW) infants but not in other neonates. Horizontal transmission occurs and can be prevented with handwashing.
Workup and Evaluation and Future Diagnostics
Workup and evaluation
Workup and evaluation for fungal infections in preterm infants includes the following tests: blood, urine, and cerebrospinal fluid (CSF) cultures. In candidemia cases, clearance of blood stream infection should be documented with two or more negative blood culture results after 3 days of treatment. As discussed earlier, due to the slow growing and adhesive properties of Candida, even in the absence of detecting end-organ dissemination, there are likely microabscesses that are slowly broken down that contribute to persistant candidemia for 3-5 days in most patients. Each negative culture result should be obtained at least 24 hours apart.
Laboratory studies at presentation
-
Obtain a complete blood cell (CBC) count with manual differential and platelet count.
-
To assess liver function, obtain aspartate amino transferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and total and direct bilirubin levels. Triglyceride levels should be included because lipid metabolism is impaired during active infections. Gamma-glutamyltransferase (GGT) levels may change with bile duct inflammation and cholestasis.
-
Measure blood urea nitrogen (BUN) and creatinine levels to assess renal function.
Laboratory studies during the infection
-
Thrombocytopenia is extremely common and can persist until clearance of Candida infection; it should be closely monitored during treatment until resolution.
-
Liver and renal function should be evaluated at the time of diagnosis (or if candidemia is persistent), because it may suggest liver or renal dissemination and the need for ultrasonography.
-
Antifungal treatment can affect serum electrolytes. The hematologic, hepatic, and renal systems and should be closely monitored during treatment.
Screening tests for dissemination
-
Screening for end-organ dissemination should be performed at the time of diagnosis in all sepsis cases and repeated if fungemia persists for longer than 5 days. Dissemination affects length of treatment.
-
The screening for dissemination includes echocardiography, renal and head ultrasonography, indirect ophthalmoscopy, and peritoneal cultures if laparotomy is performed to manage necrotizing enterocolitis (NEC) or focal bowel perforation (FBP).
Laboratory testing in patients with persistent candidemia
In patients with persistent candidemia that lasts more than 2 days, central catheters should be removed if they still remain (central catheters should be removed when bloodstream infection is diagnosed).
In patients with persistent candidemia longer than 5 days, repeat screening tests for vegetation or abscess, including the following:
-
Echocardiography
-
Ultrasonography of kidneys, head, liver, and spleen
-
Bone scan or joint aspiration (if clinical symptoms warrant)
-
Ultrasonography or laparotomy of the abdomen (in patients with a history of abdominal surgery, NEC, or FBP, to evaluate for abscesses)
-
Ultrasonography, venography, or magnetic resonance venography (MRV) of the previous location of the catheter tip (if the patient had any vascular catheters prior to or at the time of diagnosis, to evaluate for a thrombus)
Other diagnostic tests
Currently, polymerase chain reaction (PCR) and fungal markers such as beta-glucan can be extremely helpful for diagnosis and for following the response to treatment of fungal infections. PCR is useful in detecting fungal infections at other sites (urine, peritoneum, abscesses complicating NEC, or FBP), when fungal infection suspicion is high despite negative cultures. Beta-D-glucan (BDG) levels can be helpful when additional information is desired to trend treatment response: BDG levels decrease over time with antifungal therapy.
Investigators are studying molecular techniques to identify fungi (and other microorganisms) and fungal susceptibility with higher sensitivity and more rapidly than with blood cultures. Examples include PCR and DNA microarray technology. The hope is that these techniques will allow for the rapid detection of small numbers of organisms in minute volumes of blood, even after antimicrobial treatment is started.
Median BDG levels in infants with invasive candidal infections are 364 pg/mL (interquartile range [IQR]: 131-976) versus 89 pg/mL (IQR: 30-127) in noninfected neonates. [49, 50, 51] Use caution if patients have been transfused with red blood cells (RBCs) or fresh frozen plasma (FFP) as BDG levels can be significantly high for weeks (170 pg/mL; range: 65-317), as well as in those who were recently or are infected with coagulase-negative Staphylococcus (CoNS) (116 pg/mL; IQR range: 46-128). [49] Therefore, the cutoff values for neonates BDG levels are higher (>125 pg/mL) than that of adults (>80 pg/mL) due to the effect fungal colonization, other infections, and transfusions on these infants. [51]
BDG levels are less helpful in diagnosing infection, and they are unreliable in infants receiving transfusions. However, they can be most helpful to track a patient’s response to treatment as BGD levels decrease in response to antifungal therapy. [50, 51] Using BDG levels to make decisions around starting empiric antifungals needs more study, but in patients not receiving transfusions, the levels may be able to guide decisions to initiate empiric antifungal therapy for 48 hours pending culture results when performing an infection evaluation.
Fungal PCR to detect the gene for 18S ribosomal RNA (rRNA) in very low birth weight (VLBW) infants has yielded promising results but requires additional study to be used with every infection evaluation. [52] PCR results detect a broader number of infections, as they not only detect patients with candidemia but are also positive in those with Candida peritonitis and those with candiduria. [53]
In addition, investigators are examining the role of monitoring markers of fungal disease to diagnose and evaluate responses to antifungal therapy. These markers include beta-D-glucan of the cell wall, anti-Candida antibodies, D-arabinitol (candidal metabolite), and fungal chitin synthase (assessed with PCR).
Microarray technology and gene chips are being studied to rapidly determine susceptibility and resistance patterns at the time of diagnosis. These will facilitate the initiation of therapy with an appropriate antifungal agent when resistance occurs and, hopefully, improve outcomes.
Treatment, Empiric Treatment, and Prevention
Prompt initiation of systemic antifungal therapy and removal of central vascular catheters (in the setting of a bloodstream infection [BSI]) at the time of diagnosis are needed to optimize successful eradication, prevent dissemination, and improve outcomes. The image below shows antifungal mechanisms.
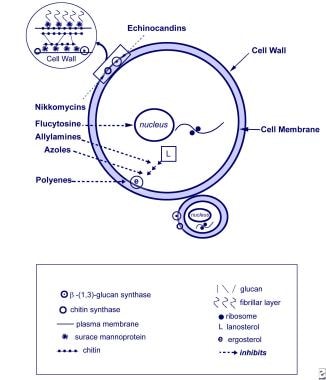 Fungal Infections in Preterm Infants. Antifungal mechanisms. Yeast cell and targets of antifungal therapy. From Kaufman 2004, with permission.
Fungal Infections in Preterm Infants. Antifungal mechanisms. Yeast cell and targets of antifungal therapy. From Kaufman 2004, with permission.
Antifungal therapy
Amphotericin
Amphotericin B deoxycholate (Fungizone) remains the primary antimicrobial medication for invasive fungal infection. This drug binds to the sterol component (ergosterol) of the cell membrane, creating a pore that leads to cell death. Although test doses have preceded administration in the past based on pediatric and adult responses to amphotericin B, this process is not necessary and delays appropriate treatment. Enough safety data now support initiating administration with a starting dose of 1 mg/kg/day without the need for lower test doses. [54, 55]
If the initial treatment is ineffective, studies have demonstrated safety with amphotericin B deoxycholate dosing increased to 1.5 mg/kg/day. [56] Poor outcomes when treating patients may be related to the delay in reaching appropriate antifungal dosing. It should be intravenously (IV) administered once daily over 2 hours. In infants receiving parenteral nutrition, a second IV line is often not needed. Parenteral nutrition can be paused, the line flushed with D5W or D10W solution; amphotericin B deoxycholate can be mixed in D10W and given in a similar volume per hour to maintain euglycemia.
Several studies have examined lipid formulations of amphotericin B, [55, 57] such as liposomal amphotericin, amphotericin B colloidal dispersion (ABCD), and amphotericin B lipid complex (ABLC).Lipid formulations distribute to the mononuclear phagocytic system, and doses of 5 mg/kg are required for efficacy similar to that of amphotericin B deoxycholate. A study that examined doses of 5-7 mg/kg of lipid amphotericin B formulations in 36 very low birth weight (VLBW) infants reported no adverse effects. [57]
A special circumstance is worth discussing. In patients with urinary tract infections (UTIs) or renal abscesses, amphotericin B deoxycholate has higher renal penetration compared with the lipid preparations and may be more effective. This is based on rodent studies that demonstrated significantly higher renal concentrations of deoxycholate, which has led most experts to recommend its preference over lipid formulations for UTIs and renal candidiasis. A rodent study examining 1 mg/kg of both amphotericin B deoxycholate and liposomal amphotericin B found kidney tissue concentrations of 735 ng/g compared with 298 ng/g, respectively. With dosing having a linear concentration for liposomal amphotericin B, a five-fold increase would be expected with a dosing of 5 mg/kg, likely resulting in similar kidney concentrations. Clinical studies are desired examining the efficacy of amphotericin products for fungal UTIs and for those with renal involvement.
There have been no studies comparing optimal dosing of 1-1.5 mg/kg of amphotericin deoxycholate (AmB-D) with 5-7 mg/kg of lipid preparations of amphotericin B.
Amphotericin resistance is extremely rare. Most C lusitaniae strains are susceptible, and infections due to these organisms clear with amphotericin. However, C lusitaniae resistance has been reported. Susceptibility testing can help guide therapeutic choices.
Azoles
Fluconazole, an azole that inhibits the enzyme C-14 lanosterol demethylase in the formation of ergosterol, has demonstrated similar efficacy to amphotericin B deoxycholate. Fluconazole has excellent tissue penetration. [58] Data recommend a dose of 12 mg/kg/day. [59, 60] Studies are also examining the need for a loading dose of 25 mg/kg; additionally, special dosing for infants on extracorporeal membrane oxygenation (ECMO) is under investigation. [61] Although these recommendations are based on pharmacokinetic data, safety of the higher dosing needs further study and close monitoring. Fluconazole is available parenterally for IV infusion or as a powder for oral suspension. The oral products are more than 90% bioavailable; therefore, the same dose may be used for oral or IV administration.
Fluconazole esistance can occur, and susceptibility testing should be performed if resistance is a concern. Most C krusei isolates are intrinsically resistant to fluconazole. As with all azoles, the drug has many potential interactions and should be closely monitored if administered with cisapride, cotrimoxazole, cyclosporine, phenytoin, rifampin, or macrolides.
Voriconazole is an azole derived from fluconazole with a broader spectrum of antifungal activity. To date, it has not been studied in neonates. Unlike fluconazole, voriconazole is 58% protein bound and contains a cyclodextrin carrier that is cleared by the kidney and can accumulate in infants with renal insufficiency. A rare complication is torsade de pointes, and 13% of pediatric patients have reported visual disturbances (ie, photophobia, blurred vision, color changes). Until further study is completed, voriconazole administration should be considered only in patients with aspergillosis. Similar to all azoles, use of voriconazole should be closely monitored if administered with cisapride or macrolides.
If there is renal insufficiency or decreased urine output, drug levels should be monitored, because pharmacokinetic studies in this population are currently lacking. Exact dosing may vary by gestational and postconceptional age and birth weight (recommend 6 mg/kg q12h for first two doses, followed by 4mg/kg/dose q12h). [62, 63]
Echinocandins
A newer class of antifungals is the echinocandins, which inhibit 1,3-beta-glucan synthesis of the cell wall. Caspofungin acetate (Cancidas) and micafungin sodium (Mycamine) are approved in the United States. These agents can be used to treat infections due to aspergillosis and with most Candida spp. Another drug in this class undergoing study is anidulafungin (Eraxis). Because these agents inhibit an enzyme, resistance and safety need to be studied along with efficacy.
Studies are under way to determine the effectiveness of echinocandins in pediatric and neonatal patients. For the echinocandins, the caspofungin dosing recommendation is around 2.5 mg/kg and micafungin dosing is around 10 mg/kg. [64, 65] In the first and only neonatal randomized controlled tria (RCT), 30 infants with invasive candidiasis were randomized 2:1 to micafungin (10 mg/kg/day) or AmB-D (1 mg/kg/day) for at least 21 days. [66] There was similar efficacy and safety with fungal-free survival 1 week after the last study drug dose in 60% of the micafungin and 70% of the AmB-D group.
Caspofungin therapy was studied in 10 neonates with candidemia that persisted 13-49 days despite treatment with amphotericin. [67] Nine infants survived, including one who had a relapse after 15 days of treatment that cleared after caspofungin was administered for another 15 days. Central venous catheters were removed as soon as blood-culture results were known. The dosage was 1 mg/kg/day for 2 days and then 2 mg/kg/day. The limitations of the study included small size, lack of pharmacokinetic data, and lack of attempted combination therapy. [67] Another study of 13 patients had a much lower success rate, with 1 mg/kg/day of caspofungin combined with other antifungals. [68] This study was complicated by delayed catheter removal. Evidence exists in these two small casposfungin studies in which the drug has shown promise and some efficacy, but its optimal dosage and safety needs further study.
Other antifungals
In the future, agents such as nikkomycins, which inhibit chitin synthase of the cell wall, may be added to the antifungal armamentarium.
Central catheter removal with candidal BSIs
Central catheter removal is critical in the treatment of neonatal candidemia. The catheter should be removed with the first positive blood culture result. Prompt removal, within 24 hours of documented positive blood culture results, is associated with lowered mortality, reduced end-organ dissemination, improved neurodevelopmental outcomes, and increased scores on the Bayley scale. [11, 12, 69] Benjamin et al demonstrated decreased mortality with prompt catheter removal and candidemia (21% vs 37%, P = 0.024) and a trend toward decreased neurodevelopmental impairment (NDI) alone (45% vs 63%, P = 0.08). [69]
While catheter removal at the time of diagnosis and replacement, if needed, after bloodstream clearance is documented is recommended, cases in which central access is required (eg, on vasopressors or peripheral access is unobtainable), removing the line and replacing it at a different site is acceptable.
Combination therapy
Amphotericin B with the addition of flucytosine (Ancobon) has been used to treat meningitis in infants who can tolerate the oral formulation of flucytosine. However, the efficacy of this regimen has not been shown to be superior to that of amphotericin B alone. Flucytosine is a fluorine analogue of cytosine that is converted to 5-fluorouracil, leading to inhibition of thymidylate synthetase and disruption of DNA synthesis. Flucytosine monotherapy rapidly leads to resistance; therefore, flucytosine cannot be used alone. For meningitis or in patients with a central nervous system (CNS) abscess, the addition of fluconazole (because of its excellent cerebrospinal fluid [CSF] penetration) is a better therapeutic option.
One study examined the use of a second antifungal agent (fluconazole) in combination with amphotericin B in patients with fungal sepsis. [55] The second agent was administered immediately upon discovery of an abscess or a positive urine culture result and also administered in patients with a persistent culture-positive infection for longer than 10 days. Infants received 1 mg/kg of amphotericin B deoxycholate (n = 34) if their creatinine level was less than 1.2 mg/dL. If the creatinine level was more than 1.2 mg/dL, they received 5 mg/kg of liposomal amphotericin B (n = 6) or ABCD (n = 14). Patients were treated for 14 days after negative culture results or until radiographic resolution of the abscess. Sterilization occurred in 36 patients (67%) with monotherapy and increased to 52 patients (96%) with polytherapy. [55]
Another concern is the treatment of presumed invasive fungal infection in the absence of positive fungus culture results. Although postmortem diagnosis of invasive candidiasis was common in the past, two studies demonstrated that only 2.7% of cases were diagnosed at autopsy. [11, 70]
Empiric treatment
In certain circumstances, empiric antifungal therapy for 48 hours may be warranted in high-risk infants with signs and symptoms of sepsis while pending culture results with one of more of the following criteria:
-
New onset thrombocytopenia (< 100 × 109/L [< 100 × 103/µL, or < 100,000/µL]) or a significant 50% decrease in the platelet count.
-
Prior exposure to third or fourth generation cephalosporins or carbapenems
-
Severe necrotizing enterocolitis (NEC) or focal bowel perforation (FBP)
-
Weight less than 750 g or a gestational age of less than 26 weeks if not on antifungal prophylaxis
In the VLBW infant, an evaluation for signs and symptoms of late-onset sepsis is typically accompanied by antibacterial treatment for at least 48 hours. Some studies have reported on the use of empiric antifungals pending culture results. Some authors propose that starting empiric antifungal therapy in infants who are not receiving antifungal prophylaxis while culture results are pending may reduce the high mortality associated with candidemia in VLBW infants, especially those born at less than 28 weeks' gestation. [9, 27] In other studies, empiric therapy has improved outcomes in critically ill VLBW infants with multiple risk factors. [71] Neither approach has been tested in a randomized controlled trial.
In a prospective study, empiric antifungal therapy (amphotericin B deoxycholate) was initiated in infants weighing less than 1500 g or "very ill” infants if three critica were met: (1) clinical signs of infection, (2) exposure to either vancomycin or a third-generation cephalosporin for 7 days, and (3) one of the following: parenteral nutrition, mechanical ventilation, postnatal steroids, H2 blocker, or candidal rash or thrush. [71] In this small study, empiric antifungal therapy eliminated candida-related mortality in these high-risk patients.
A scoring system has been proposed that includes thrombocytopenia, gestational age of less than 28 weeks, and broad-spectrum antibiotic treatment; however, this system has not been prospectively studied for safety or efficacy. [9] Infants with NEC or FBP are also at increased risk. Further study is needed to investigate the efficacy and safety of empiric antifungal therapy.
Most fungi are isolated from cultures within 48 hours. [72] Therefore, some experts do not recommend empiric antifungal therapy. They recommend prompt initiation of antifungal treatment and removal of any central venous catheters upon positive culture results. In a study by Noyola et al, the start of antifungal therapy and the removal of central vascular catheters within 2 days after blood cultures were obtained was not associated with increased morbidity or mortality in episodes of fungal sepsis. [11]
Preemptive treatment
Preemptive treatment has been used in a few neonatal studies following early detection of endotracheal or respiratory Candida colonization by culture or mannan antigen in infants 28 weeks’ gestation or younger. Respiratory colonization is a high-risk site in preterm infants. [73, 74] In a study that used the approach of early identification of Candida spp from tracheal aspirates followed by 14 days of antifungal treatment, invasive Candida infections were reduced from 75% (12 of 16 with positive growth of Candida in culture) to 0% (0 of 16 with Candida mannan detected). [75] This approach was associated with no Candida -associated mortality. Another group that might benefit from preemptive treatment is patients with NEC in whom Candida is isolated from their gastrointestinal tract.
Prevention
See the table below.
Table 2: Evidenced-Based Measures to Prevent Invasive Candida Infections (Open Table in a new window)
1. Targeted antifungal prophylaxis with intravenous (IV) fluconazole for the following infants:
|
2. Promptly treat congenital cutaneous candidiasis (A-II)
|
2. Decrease broad-spectrum antibiotic use (A-II)
|
3. Decrease acid inhibitor use (H2 blockers or proton pump inhibitors) (B-II)
|
4. Decrease postnatal dexamethasone use (B-II)
|
| 5. Central line-associated bloodstream infection (BSI) reduction bundles (A-II) |
| 6. Monitor rates of all ICI including BSI, UTI, meningitis, and peritonitis. Provide feedback to all neonatal teams (B-II). |
7. Infection control measures (A-II)
|
US Public Health Service Grading System for ranking recommendations in clinical guidelines: Strength of recommendation and levels of evidence. A: good evidence; B: moderate evidence; C: poor evidence. I: at least one randomized clinical trial; II: at least one well-designed but nonrandomized trial; III: expert opinions based on experience or limited clinical reports. ICI = invasive Candida Infections; IVF = intravenous fluids; UTI = Urinary tract infection. |
Prophylaxis
Because of the high mortality and neurodevelopmental impairment (NDI) associated with fungal sepsis in VLBW infants, prevention with fluconazole studies and a few nystatin studies have been performed in the highest-risk patients.
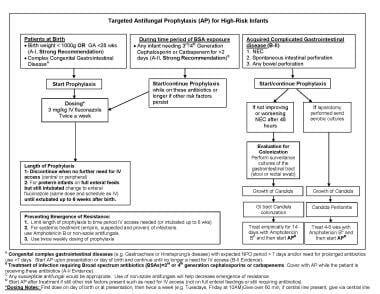 Fungal Infections in Preterm Infants. Fungal Infections in Preterm Infants. Targeted antifungal prophylaxis. (A) Acquired complicated or congenital gastrointestinal diseases (eg, gastroschisis or Hirshsprung disease) with expected nil per os (NPO) period longer than 7 days and/or need for prolonged antibiotics use longer than 7 days. Start antifungal prophylaxis (AP) upon presentation or day of birth and continue until there is no longer a need for intravenous (IV) access (B-II Evidence). (B) Treatment of infection requiring broad spectrum antibiotics (BSAs) = 3rd or 4th generation cephalosporins or carbapenems. Cover with AP while the patient is receiving these antibiotics (A-II Evidence). (C) For any bowel perforation with or without necrotizing enterocolitis (NEC), if laparotomy is performed, send aerobic cultures to evaluate for fungal peritonitis and treat 4-6 weeks if present. *Dosing notes: First dose on day of birth, then twice a week (for example: Tuesdays, Friday at 10AM). Give over 60 minutes; if central line present, give via central line.
Fungal Infections in Preterm Infants. Fungal Infections in Preterm Infants. Targeted antifungal prophylaxis. (A) Acquired complicated or congenital gastrointestinal diseases (eg, gastroschisis or Hirshsprung disease) with expected nil per os (NPO) period longer than 7 days and/or need for prolonged antibiotics use longer than 7 days. Start antifungal prophylaxis (AP) upon presentation or day of birth and continue until there is no longer a need for intravenous (IV) access (B-II Evidence). (B) Treatment of infection requiring broad spectrum antibiotics (BSAs) = 3rd or 4th generation cephalosporins or carbapenems. Cover with AP while the patient is receiving these antibiotics (A-II Evidence). (C) For any bowel perforation with or without necrotizing enterocolitis (NEC), if laparotomy is performed, send aerobic cultures to evaluate for fungal peritonitis and treat 4-6 weeks if present. *Dosing notes: First dose on day of birth, then twice a week (for example: Tuesdays, Friday at 10AM). Give over 60 minutes; if central line present, give via central line.
Fluconazole studies
Fluconazole is an excellent drug for prophylaxis because of its long half-life, high tissue concentration, low lipophilicity, and low protein binding. One concern with fluconazole prophylaxis through the years has been the emergence of resistance over time, but with dosing of 3 mg/kg twice while high-risk patients have central or peripheral access, resistance has not been seen after several years of prophylaxis.
The initial randomized controlled trial of IV fluconazole prophylaxis was performed in high-risk infants who weighed less than 1000 g and had an endotracheal tube or central vascular catheter; it was effective in preventing invasive fungal infection. [29] Investigators studied prophylaxis using 3 mg/kg of IV fluconazole every 72 hours on days 1-14, every 48 hours on days 15-28, and then daily administration on days 29-42 for as long as 6 weeks if IV access is not required. No adverse effect or fungal resistance was detected during the 30-month study period. [29] The same authors examined dosing with 3 mg/kg twice a week compared with the regimen described above and found similar efficacy. [76]
Manzoni and colleagues investigated two different fluconazole doses (3 mg/kg and 6 mg/kg) compared with a placebo group in 322 Italian infants who weighed less than 1500 g and reported a significant difference between the fluconazole prophylaxis groups compared with the placebo patients. [77] Fluconazole prophylaxis had similar efficacy for both the 3 mg/kg and 6 mg/kg groups. Fungal colonization occurred in 7.7% and 9.8% and invasive fungal infection in 3.8% and 2.7% in the 3 mg/kg and 6 mg/kg groups, respectively. In the placebo group, fungal colonization occurred in 29.2% and invasive infection occurred in 13.2% (P = .005 for 6 mg; P = .02 for 3 mg). [77] The results also showed a significant effect in infants less than 1000 g as well as infants less than 27 weeks' gestation.
Another important trial in infants weighing less than 750 g demonstrated a statistically significant decrease in invasive candidiasis in the fluconazole-treated patients compared with the placebo group (9% vs 3%). [78] However, the study was underpowered to examine the primary outcome of infection and all-cause mortality. The composite outcome of invasive infection or death would need approximately 1200 patients to be appropriately powered compared with the 361 infants studied. The study did show a high 41% mortality in this patient population who developed invasive Candida infections, compared to 18% in patients without candidiasis. [78]
The largest multicenter case-controlled analysis of efficacy and safety included 95 neonatal intensive care units (NICUs) that also demonstrated efficacy and safety for fluconazole prophylaxis. [79] Fluconazole prophylaxis was administered to 127 patients [754 ± 163 g birth weight (BW) and 25.4 ± 1.7 weeks' gestational age (GA)] and were compared to 399 control patients (756 ± 163 g BW and 25.5 ± 1.8 weeks GA). Invasive Candida infection occurred in 1 (0.8%) of 127 infants who received fluconazole prophylaxis compared to 29 (7.3%) of 399 of the matched controls (P = .006). Candida BSI occurred in 1 (0.8%) of 127 fluconazole prophylaxis infants compared to 22 (5.5%) of 399 of the matched controls (P = .02). There were no differences in late-onset sepsis due to gram-positive or gram-negative organisms, FBP, NEC, cholestasis, or overall mortality. [79]
Several observational studies examined fluconazole prophylaxis in both extremely low birth weight (ELBW) and VLBW infants. [74, 80, 81, 74] A meta-analysis of randomized and observational studies with control subjects using Mantel-Haenszel methods demonstrated an 84% reduction in invasive fungal infections among 2111 preterm infants. [26, 29, 74, 80, 81, 82] For high-risk ELBW infants, the studies demonstrated an 88% reduction in invasive fungal infections.
Dosing with 3 mg/kg twice weekly is effective and limits exposure, cost, and potential adverse effects. When initiated around birth, prophylaxis should be administered for 6 weeks or less in patients with a birth weight below 1000 g or less than 6 weeks if IV access is no longer needed. For patients with a birth weight of more than 1000 g, continue prophylaxis until IV access is no longer needed and until adequate enteral feedings are achieved.
If antifungal prophylaxis with fluconazole is administered, it is important to use a different antifungal agent (eg, amphotericin B) for primary treatment of an invasive fungal infection. This ensures treatment with a susceptible antifungal agent and possibly decreases the risk of fungal resistance. Surveillance cultures for fungal resistance are recommended when fluconazole prophylaxis is completed.
Azole resistance
Fluconzole prophylaxis has been safe and studies have not demonstrated resistance with dosing of 3 or 6 mg/kg twice a week for over 20 years. [29, 76, 83, 84] Studies of minimum inhibitory concentration (MIC) have demonstrated no significant resistance among fungal strains causing colonization or infection over 5-, 6-, and 11-year time periods in various NICUs. These studies have also shown a relationship with the frequency of dosing and fungal resistance. Twice weekly dosing of 3 m/kg compared to more frequent dosing of fluconazole prophylaxis was associated with significantly less colonization with resistant fungal isolates. [29, 76] A retrospective evaluation of all clinical and surveillance fungal isolates obtained from infants during over a 22-year period (6 years prior to and 16 years after instituting fluconazole prophylaxis) revealed no significant evidence of colonization or infection with C glabrata or C krusei strains. [85] In evaluating over 1400 VLBW infants, the rate of C glabrata or C krusei colonization remained approximately 4% and the infection rate below 1%, and no significant differences were detected when comparing the two study periods. It is important to note that in all these fluconazole prophylaxis studies, fungal infections were treated with amphotericin B preparations, which decreases the overall exposure to fluconazole and may intermittently eliminate resistant organisms.
Sarvikivi et al used fluconazole prophylaxis over a 12-year period (1991–2000) in infants weighing less than 1000 g and who were below 30 weeks' gestation, and reported minimal resistance. After 10 years of targeted prophylaxis in preterm infants, the NICU expanded their protocol to the entire NICU and increased the daily dose to 6-12 mg/kg. Within 2 years, they reported two infants who developed a fluconazole resistant strain of C parapsilosis. This study and others have revealed a higher risk of emergence of resistance with higher (≥6 mg/kg) and more frequent (daily) dosing of fluconazole.
Safety
In addition to dosing and frequency of fluconaozle prophylaxis attenuating the emergence of resistance, studies have demonstrated short- and long-term safety with fluconazole prophylaxis. There is no adverse effect on liver function or long-term neurodevelopmental outcomes at 18-22 months or 8-10 years. [78, 86]
Nystatin studies
Nystatin, an enteral or orally administered nonabsorbable antifungal agent, was the first antifungal studied for prophylaxis in preterm infants. Nystatin studies have added to our understanding of antifungal prophylaxis by demonstrating its efficacy and use in NICUs with low rates of infection, revealing the greater efficacy of prophylaxis when it is started early (by 72 hours after birth) versus later when colonization is detected, and a decrease in all-cause mortality.
Antifungal prophylaxis started after colonization is detected is not as effective as starting shortly after birth in preventing infections. In a study by Ozturk et al, fungal BSI was decreased by 90% when nystatin prophylaxis was started in the first 72 hours (47 ± 12 hours, mean ± SD) compared with only 62% when started after colonization was detected (12.6 ± 2.4 days). [87]
Two large retrospective studies of nystatin prophylaxis demonstrated the use of antifungal prophylaxis even in NICUs with low Candida bloodstream rates. In the largest multicenter epidemiologic study to date (N = 14,778), NICUs using nystatin prophylaxis had lower rates of BSI or meningitis in infants weighing less than 1500 g (0.54% vs 1.23%, P < .0001) and in those weighing less than 1000 g (1.23% vs 2.67%, P < .0001) compared to NICUs that did not use antifungal prophylaxis. [88]
The second large retrospective study of nystatin (1459 infants) demonstrated similar efficacy of nystatin prophylaxis in reducing invasive candidal infections in preterm infants younger than 33 weeks. [89] There was a 56% decrease in such infections, from 4.1% to 1.8%, and a 54% decrease of invasive candidal infections in infants weighing less than 1500 grams (5.5% to 2.5%) with nystatin prophylaxis. This study also found that all-cause mortality was lower in the nystatin prophylaxis group (11.8%) compared to patients not receiving prophylaxis (17.8%) (P < .0001). [89]
Fluconazole versus nystatin prophylaxis.
Studies have demonstrated greater efficacy with fluconazole prophylaxis (80%) compared to around 50% with nystatin prophylaxis. [4, 90, 91] Futuremore, fluconazole prophyalxis studies have an overall higher level of evidence and more studies from randomized controlled trials. Additionally, fluconaole prophylaxis is given IV, allowing the prophylaxis be delivered to extremely preterm infants who may not tolerate enteral feedings in the first weeks after birth when they are at highest risk for invasive candidiasis and its Candida-related mortality. Twice weekly adminstration of fluconazole prophylaxis compared to three to four times a day nystatin prophylaxis is often favorable from systems managment, decreasing pharmacy and nursing time, and is less expensive over the course of prophylaxis.
Who is at high risk and should receive antifungal prophylaxis?
Because prophylaxis works best if initiated in the first days of life, identifying such infants at that time leads to the greatest effect in reducing infection. One study of infants weighing less than 1250 g in 95 NICUs examined maternal and neonatal risk factors present at birth. [92] NICUs can examine their invasive Candida infection rates at each gestational age by week to see at what gestational age infections fall to zero. From published data including all invasive Candida infections (BSIs, UTIs, peritonitis, and meningitis), antifungal prophylaxis would be beneficial for infants born at less than 28 weeks' gestation while they have risk factors (see below). [9, 30]
Prophylaxis should be administered while high-risk infants require IV access. This targets prevention to the time period infants have risk factors. When IV access is no longer required, the risk for invasive fungal infection falls, because the additional risk factors for fungemia (eg, parenteral nutrition, lipid emulsions, broad-spectrum antibiotics, and central venous access) are no longer present. [14, 29] For example, a 26-weeks' gestation infant who reaches full enteral feedings at 3 weeks after birth (and no longer needs central or peripheral IV access), would receive only six doses of fluconazole. Other patients that may benefit and are being studied include preterm and full-term infants with complicated gastrointestinal disease (eg, NEC, gastroschisis) who require prolonged periods without enteral feedings.
The American Academy of Pediatrics (AAP) advocates for prophylaxis in the highest-risk patients (birth weight < 1000 g). [93, 94] The highest-risk patients who may benefit include the following:
-
All infants with a birth weight of less than 1000 g and/or younger than 28 weeks' gestation
-
Preterm and full-term infants with gastrointestinal disease, such as NEC, FBP, or complicated cases of gastroschisis, omphalocele, intestinal atresia, and Hirschsprung disease (eg, exposed to antibiotics and not receiving or expected to receive enteral feedings for >7 days)
For patients who receive fluconazole prophylaxis, the dose should be 3 mg/kg twice weekly until IV access is no longer required. In patients with suspected or documented fungal infection who receive prophylaxis, amphotericin B should be administered as the initial antifungal therapy until Candida spp infection and fungal susceptibility is determined.
Morbidity, Mortality, and Follow-up
Morbidity
Neurodevelopmental impairment (NDI) is more common in infants with fungal sepsis who weigh less than 1000 g than in extremely low birth weight (ELBW) infants without infection. [38, 69, 95] NDI does not appear to be more common in infants who weigh 1000-1500 g, but a more detailed study of this subgroup is needed.
In a study of NDI associated with these infections in ELBW infants, Stoll et al examined mental and psychomotor developmental indexes, cerebral palsy (CP), and hearing or visual impairment. [95] There was at least one adverse neurodevelopmental outcome in 41% of infected infants (any clinical sepsis, bloodstream infection, or meningitis) and 57% of infants with fungal sepsis. The prevalence of adverse neurodevelopmental outcomes in infants with fungal sepsis were as follows:
-
Mental developmental index of less than 70: 34%
-
Psychomotor developmental index below 70: 24%
-
CP: 18%
-
Visual impairment: 14%
-
Hearing impairment: 5%
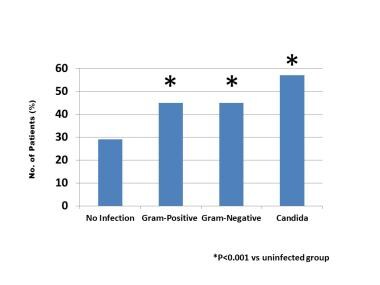 Fungal Infections in Preterm Infants. Neurodevelopmental impairment in infants weighing below 1000 grams with bloodstream infection.
Fungal Infections in Preterm Infants. Neurodevelopmental impairment in infants weighing below 1000 grams with bloodstream infection.
The rate of NDI in infants with fungal sepsis did not differ significantly from those with bloodstream infection with other microorganisms (coagulase-negative staphylococcus [CONS], non-CONS, and gram-positive and gram-negative organisms). [95]
The effect of invasive fungal infection on other morbidities is still being studied. In ELBW infants, several studies have described an association with retinopathy of prematurity. One study demonstrated an increased incidence of bronchopulmonary dysplasia. [38]
Because of infants' maturing immune systems, outcomes may better correlate with corrected gestational age at the time of infection. Infants who develop infection later in their hospital stay (ie, after 6 weeks) may have better outcomes, but this requires further study.
Mortality
In ELBW infants, candidemia is associated with significant mortality (26%) compared to infants weighing more than1000 grams (2%). [69, 96, 97] Less than 26 weeks' gestation and a birth weight of below 750 g correlate with increased mortality (40%). [78] Mortality is significantly higher when sepsis is due to C albicans (nearly 44%) than when it is due to C parapsilosis (15%) or other Candida spp. [98] Of course, keep in mind that results vary from center to center, and several single-center studies have reported no mortality; thus, intensive care management of the septic infant and other factors may play an important role in survival. [1, 27, 97]
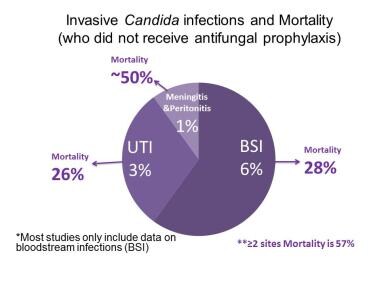 Fungal Infections in Preterm Infants. Mortality associated with infants weighing less than 1000 grams with invasive Candida infections. Bloodstream and urinary tract infections have similar high mortality, and mortality increases to 50% or greater for infections involving multiple sites, peritonitis, or meningitis. Source of data: Benjamin DK Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010 Oct. 126 (4):e865-73.
Fungal Infections in Preterm Infants. Mortality associated with infants weighing less than 1000 grams with invasive Candida infections. Bloodstream and urinary tract infections have similar high mortality, and mortality increases to 50% or greater for infections involving multiple sites, peritonitis, or meningitis. Source of data: Benjamin DK Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010 Oct. 126 (4):e865-73.
Follow-up
All preterm infants with infection should receive neurodevelopmental follow-up in the first few years of life and early intervention services, if needed.
-
Fungal Infections in Preterm Infants. Pathogenesis and invasive fungal infections in very low birth weight infants. From Kaufman and Fairchild 2004, with permission.
-
Fungal Infections in Preterm Infants. Percutaneous intravenous central catheter and fungal biofilm formation.
-
Fungal Infections in Preterm Infants. Risk factors for candidemia. PICC = peripherally inserted central catheter.
-
Fungal Infections in Preterm Infants. Congenital cutaneous candidiasis in a 26-week-old infant.
-
Fungal Infections in Preterm Infants. Hepatic candidiasis. Note the white pustules of Candida albicans on the surface of the liver.
-
Fungal Infections in Preterm Infants. Antifungal mechanisms. Yeast cell and targets of antifungal therapy. From Kaufman 2004, with permission.
-
Fungal Infections in Preterm Infants. Mortality associated with infants weighing less than 1000 grams with invasive Candida infections. Bloodstream and urinary tract infections have similar high mortality, and mortality increases to 50% or greater for infections involving multiple sites, peritonitis, or meningitis. Source of data: Benjamin DK Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010 Oct. 126 (4):e865-73.
-
Fungal Infections in Preterm Infants. Neurodevelopmental impairment in infants weighing below 1000 grams with bloodstream infection.
-
Fungal Infections in Preterm Infants. Fungal Infections in Preterm Infants. Targeted antifungal prophylaxis. (A) Acquired complicated or congenital gastrointestinal diseases (eg, gastroschisis or Hirshsprung disease) with expected nil per os (NPO) period longer than 7 days and/or need for prolonged antibiotics use longer than 7 days. Start antifungal prophylaxis (AP) upon presentation or day of birth and continue until there is no longer a need for intravenous (IV) access (B-II Evidence). (B) Treatment of infection requiring broad spectrum antibiotics (BSAs) = 3rd or 4th generation cephalosporins or carbapenems. Cover with AP while the patient is receiving these antibiotics (A-II Evidence). (C) For any bowel perforation with or without necrotizing enterocolitis (NEC), if laparotomy is performed, send aerobic cultures to evaluate for fungal peritonitis and treat 4-6 weeks if present. *Dosing notes: First dose on day of birth, then twice a week (for example: Tuesdays, Friday at 10AM). Give over 60 minutes; if central line present, give via central line.
Tables
What would you like to print?
- Overview
- Pathogenesis
- Risk Factors
- Candidal Infections
- Clinical Presentation and Differential Diagnosis
- Aspergillosis, Mucormycosis (Zygomycosis) and Malassezia Infections
- Workup and Evaluation and Future Diagnostics
- Treatment, Empiric Treatment, and Prevention
- Morbidity, Mortality, and Follow-up
- Show All
- Media Gallery
- Tables
- References