Practice Essentials
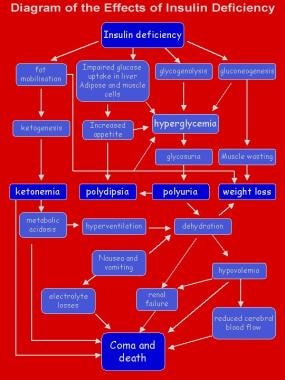
Type 1 diabetes mellitus is a chronic illness characterized by the body’s inability to produce insulin due to the autoimmune destruction of the beta cells in the pancreas. Most pediatric patients with diabetes have type 1 and a lifetime dependence on exogenous insulin. [1] The image below depicts the effects of insulin deficiency.
Signs and symptoms
Signs and symptoms of type 1 diabetes mellitus in children include the following:
-
Hyperglycemia
-
Glycosuria
-
Polydipsia
-
Polyuria
-
Unexplained weight loss
-
Nonspecific malaise
-
Symptoms of ketoacidosis - Abdominal pain, nausea, vomiting, and abnormal breathing
See Presentation for more detail.
Diagnosis
Antibody testing:
Type 1 diabetes can be diagnosed pre-clinically (before onset of dysglycemia or hyperglycemia) by screening with type 1 diabetes mellitus antibodies (zinc transporter 8, glutamic acid decarboxylase-65 [GAD65], islet cell, islet antigen 2 [IA2], insulin autoantibodies [IAAs]). Two or more positive antibodies are diagnostic of type 1 diabetes mellitus. [2]
Blood glucose
Blood glucose tests using capillary blood samples, reagent sticks, and blood glucose meters are the usual methods for monitoring day-to-day diabetes control. Continuous glucose monitoring has become standard of care for all pediatric patients with diabetes on insulin.
Diagnostic criteria by the American Diabetes Association (ADA) include the following [2] :
-
Hemoglobin A1c (HbA1c) ≥ 6.5% (≥48 mmol/mol)
-
A fasting plasma glucose (FPG) level ≥126 mg/dL (7.0 mmol/L), or
-
A 2-hour plasma glucose level ≥200 mg/dL (11.1 mmol/L) during a 75-g oral glucose tolerance test (OGTT), or
-
A random plasma glucose ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis
See Workup for more detail.
Management
Glycemic control
The American Diabetes Association (ADA) recommends assessing glycemic control using HbA1c and/or appropriate continuous glucose monitoring metrics every 3 months in pediatric patients who are growing. [3] HbA1c represents an average glucose level but may be impacted by factors such as increased red cell turnover. Continuous glucose monitoring is frequently used in persons with type 1 diabetes mellitus and those who have risk of hypoglycemia, which may alter HbA1c testing.
Goals of glycemic control (regardless of age) are HbA1c of less than 7% for most patients. If using continuous glucose monitoring, this should be interpreted over a 14-day period and used in addition to HbA1c monitoring.
Individualization of the HbA1c goal may be appropriate for certain pediatric patients, such as those with limited ability to articulate hypoglycemia or at risk for severe hypoglycemia (younger children or pre-verbal infants), those with a history of severe hypoglycemia, and those with limited life expectancy. In these patients, an HbA1c goal of 7.5% or 8% may be appropriate. [4, 5]
Likewise, according to a modified recommendation for children and adolescents in the ADA's Standards of Care in Diabetes—2025, more stringent HbA1c goals (eg, < 6.5% [< 48 mmol/mol]) can reasonably be suggested by health-care professionals for selected patients if these goals can be reached “without significant hypoglycemia, excessive weight gain, negative impacts on well-being, or undue burden of care or in those who have nonglycemic factors that decrease A1C (eg, lower erythrocyte life span)." During the honeymoon phase, according to the recommendation, lower goals may also be suitable. [6, 7]
Insulin therapy
All children with type 1 diabetes mellitus require insulin therapy with either multiple daily dosing of subcutaneous injections (MDI) or continuous insulin infusion/insulin pumps. MDI regimens consist of a basal (long- or intermediate-acting) insulin and a preprandial (short-acting, premeal) insulin, as well as insulin given to correct hyperglycemia as needed (short acting). Long-acting basal insulin includes glargine and detemir, while intermediate-acting insulin includes neutral protamine Hagedorn (NPH). Rapid-acting preprandial insulin includes lispro, aspart, and glulisine.
The ADA recommends that insulin pump therapy be offered to all pediatric patients with type 1 diabetes mellitus who have the ability for and are in agreement with use of the technology. [4, 5]
Diet and activity
The aim of dietary management is to balance the child's food intake with insulin dose and activity and to keep blood glucose concentrations as close as possible to reference ranges, avoiding extremes of hyperglycemia and hypoglycemia.
The following are among the most recent dietary consensus recommendations (although they should be viewed in the context of the patient’s culture) [8] :
-
Carbohydrates - Should provide 50-55% of daily energy intake; no more than 10% of carbohydrates should be from sucrose or other refined carbohydrates
-
Fat - Should provide 30-35% of daily energy intake
-
Protein - Should provide 10-15% of daily energy intake
Exercise is also an important aspect of diabetes management. It has real benefits for a child with diabetes. Patients should be encouraged to exercise regularly.
See Treatment and Medication for more detail.
Background
Most pediatric patients with diabetes have type 1 diabetes mellitus and a lifetime dependence on exogenous insulin. Diabetes mellitus (DM) is a chronic metabolic disorder caused by an absolute or relative deficiency of insulin, an anabolic hormone. Insulin is produced by the beta cells of the islets of Langerhans, located in the pancreas. The absence of these cells or their loss through autoimmune destruction or other means results in type 1 diabetes mellitus. The most common cause of type 1 diabetes mellitus is the autoimmune destruction of insulin-producing cells. The development of autoimmune type 1 diabetes mellitus follows three stages [9] :
-
Stage I - Autoimmunity develops; lab analysis shows positive antibodies, but the patient has normoglycemia; this stage is asymptomatic/presymptomatic
-
Stage II - Dysglycemia/abnormal glucose tolerance develops, but the patient remains asymptomatic
-
Stage III - Overt hyperglycemia and symptoms of type 1 diabetes
Type 2 diabetes mellitus is a heterogeneous disorder. Patients with type 2 diabetes mellitus have insulin resistance and their beta cells lack the ability to overcome this resistance. [10] Although this form of diabetes was previously uncommon in children, the prevalence is increasing in youth. Other patients may have inherited disorders of insulin release (monogenic diabetes, ie, maturity-onset diabetes of the young [MODY]). [11, 12, 13] This topic addresses only type 1 diabetes mellitus.
Hypoglycemia
Hypoglycemia occurs secondary to exogenous insulin use in the treatment of type 1 diabetes mellitus and can be acutely dangerous or life threatening. [14]
Manage mild hypoglycemia by giving rapidly absorbed oral carbohydrate or glucose; for a patient unable to take oral glucose, administer glucagon. Glucagon stimulates the release of liver glycogen and releases glucose into the circulation. Where appropriate, an alternative therapy is intravenous (IV) glucose (preferably no more than a 10% glucose solution). All treatments for hypoglycemia provide recovery in approximately 10 minutes. (See Treatment.)
Hyperglycemia
In an otherwise healthy individual, blood glucose levels rarely rise above 180 mg/dL (9 mmol/L). In a child with diabetes, blood sugar levels rise if insulin is insufficient for a given glucose load. The renal threshold for glucose reabsorption is exceeded when blood glucose levels are above 180 mg/dL (10 mmol/L), causing glycosuria (with the typical symptoms of polyuria and polydipsia). (See Pathophysiology and Treatment.)
All children with diabetes experience episodes of hyperglycemia, but persistent hyperglycemia may lead to long-term complications.
Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a life-threatening medical emergency. [15] Ketosis usually does not occur when insulin is present. In the absence of insulin, severe hyperglycemia, dehydration, and ketone production contribute to the development of DKA. The most serious complication of DKA is the development of cerebral edema, which increases the risk of long-term morbidity and death. Very young children at the time of first diagnosis are most likely to develop cerebral edema.
DKA usually follows increasing hyperglycemia and symptoms of osmotic diuresis. Users of insulin pumps, by virtue of absent reservoirs of long-acting subcutaneous insulin, may present with euglycemic ketosis/DKA. They are more likely to present with nausea, vomiting, and abdominal pain, symptoms similar to food poisoning. DKA may also manifest as respiratory distress.
Injection-site hypertrophy
If children persistently inject their insulin into the same area, subcutaneous tissue swelling may develop, adversely affecting insulin absorption. Rotating the injection sites helps to prevent this complication.
Fat atrophy can also occur, possibly in association with insulin antibodies. This condition is much less common but is more disfiguring.
Diabetic retinopathy
Although it is the most common cause of acquired blindness in many developed nations, diabetic retinopathy is rare in the prepubertal child or within 5 years of the onset of diabetes. The prevalence and severity of retinopathy increase with age and are greatest in patients whose diabetic control is poor. [16] Prevalence rates seem to be declining, with studies showing a 30% prevalence of diabetic retinopathy in those with type 1 diabetes mellitus, compared with previous studies citing upwards of 97% prevalence. [17]
Diabetic nephropathy and hypertension
The exact mechanism of diabetic nephropathy is unknown. Peak incidence is in post adolescence, 10-15 years after diagnosis, and it may occur in as many as 21% of people with type 1 diabetes mellitus. [18]
In a patient with nephropathy, the albumin excretion rate (AER) increases until frank proteinuria develops, and this may progress to renal failure. Blood pressure rises with increased AER, and hypertension accelerates the progression to renal failure. Having diabetic nephropathy also increases the risk for significant diabetic retinopathy.
Progression may be delayed or halted by improved diabetes control, administration of angiotensin-converting enzyme (ACE) inhibitors, and aggressive blood pressure control. Regular urine screening for microalbuminuria provides opportunities for early identification and treatment to prevent renal failure.
A child younger than 15 years with persistent proteinuria may have a nondiabetic cause and should be referred to a pediatric nephrologist for further assessment.
Peripheral and autonomic neuropathy
The peripheral and autonomic nerves are affected in type 1 diabetes mellitus. [19] Hyperglycemic effects on axons and microvascular changes in endoneural capillaries are amongst the proposed mechanisms. (In adults, peripheral neuropathy usually occurs as a distal sensory loss.)
Autonomic changes involving cardiovascular control (eg, heart rate, postural responses) have been described in as many as 40% of children with diabetes. Cardiovascular control changes become more likely with increasing duration and worsening control. [20] In a study by 253 patients with type 1 diabetes (mean age at baseline 14.4 y), Cho et al reported that the prevalence of cardiac autonomic dysfunction increases in association with higher body mass index and central adiposity. [21]
Another complication, gastroparesis, may be caused by autonomic dysfunction. Gastric emptying is significantly delayed, leading to problems of bloating and unpredictable excursions of blood glucose levels.
Macrovascular disease
Although this complication is not commonly seen in pediatric patients, it is a significant cause of morbidity and premature mortality in adults with diabetes. People with type 1 diabetes mellitus have twice the risk of fatal myocardial infarction (MI) and stroke compared with people unaffected with diabetes; in women, the MI risk is 4 times greater. People with type 1 diabetes mellitus also have four times greater risk for atherosclerosis.
The combination of peripheral vascular disease and peripheral neuropathy can cause serious foot pathology. Smoking, hypertension, hyperlipidemia, and poor diabetic control greatly increase the risk of vascular disease. Smoking, in particular, may increase the risk of myocardial infarction by a factor of 10.
Autoimmune diseases
Hypothyroidism occurs in approximately 1-2% of all patients with diabetes, with significantly higher rates in those with type 1 diabetes mellitus. The prevalence of hypothyroidism in females with type 1 diabetes mellitus is estimated to be 12-24%, while in males with type 1 diabetes mellitus, the prevalence is about 6%. [22]
Although Addison disease is uncommon, affecting less than 1% of children with diabetes, it is a life-threatening condition that is easily missed. Addison disease may reduce the insulin requirement and increase the frequency of hypoglycemia.
Celiac disease, associated with an abnormal sensitivity to gluten in wheat products, is an autoimmune disease that may occur in as many as 5% of children with type 1 diabetes mellitus. [23]
Limited joint mobility
Limited joint mobility (primarily affecting the hands and feet) is believed to be associated with poor diabetic control. [24]
Originally described in approximately 30% of patients with type 1 diabetes mellitus, limited joint mobility occurs in 50% of patients older than age 10 years who have had diabetes for longer than 5 years. The condition restricts joint extension, making it difficult to press the hands flat against each other. The skin of patients with severe joint involvement has a thickened and waxy appearance.
Limited joint mobility is associated with increased risks for diabetic retinopathy and nephropathy. Improved diabetes control over the past several years appears to have reduced the frequency of these additional complications by a factor of approximately four. Patients have also markedly fewer severe joint mobility limitations.
Pathophysiology
Insulin is essential to process carbohydrates, fat, and protein. Insulin reduces blood glucose levels by allowing glucose to enter muscle cells and by stimulating the conversion of glucose to glycogen (glycogenesis) as a carbohydrate store. Insulin also inhibits the release of stored glucose from liver glycogen (glycogenolysis) and slows the breakdown of fat to triglycerides, free fatty acids, and ketones. In addition, it stimulates fat storage. Insulin also inhibits the breakdown of protein and fat for glucose production (gluconeogenesis) in the liver and kidneys.
Hyperglycemia
Hyperglycemia results when insulin deficiency leads to uninhibited gluconeogenesis and prevents the use and storage of circulating glucose. The kidneys cannot reabsorb the excess glucose load, causing glycosuria, osmotic diuresis, thirst, and dehydration. Increased fat and protein breakdown leads to ketone production and weight loss. Without insulin, a child with type 1 diabetes mellitus wastes away and eventually dies due to DKA. The effects of insulin deficiency are shown in the image below.
Hypoglycemia
Insulin inhibits gluconeogenesis and glycogenolysis, while stimulating glucose uptake. In individuals without diabetes, insulin production by the pancreatic islet cells is suppressed when blood glucose levels fall below 83 mg/dL (4.6 mmol/L). If insulin is injected into a treated child with diabetes who has not eaten adequate amounts of carbohydrates, blood glucose levels progressively fall.
The brain depends on glucose as a fuel. As glucose levels drop below 65 mg/dL (3.2 mmol/L), counterregulatory hormones (eg, glucagon, cortisol, epinephrine) are released, and symptoms of hypoglycemia develop. These symptoms include sweatiness, shaking, confusion, behavioral changes, and, eventually, coma.
The glucose level at which symptoms develop varies greatly from individual to individual (and from time to time in the same individual), depending in part on the duration of diabetes, the frequency of hypoglycemic episodes, the rate of fall of glycemia, and overall control.
Etiology
Most cases (95%) of type 1 diabetes mellitus are the result of environmental factors interacting with a genetically susceptible person. This interaction leads to the development of autoimmune disease directed at the insulin-producing cells of the pancreatic islets of Langerhans. These cells are progressively destroyed, with insulin deficiency usually developing after the destruction of 90% of islet cells.
Genetic issues
Clear evidence suggests a genetic component in type 1 diabetes mellitus. Monozygotic twins have a 60% lifetime concordance for developing type 1 diabetes mellitus, although only 30% do so within 10 years after the first twin is diagnosed. In contrast, dizygotic twins have only an 8% risk of concordance, which is similar to the risk among other siblings.
The frequency of diabetes development in children with a mother who has diabetes is 2-3%; this figure increases to 5-6% for children with a father who has type 1 diabetes mellitus. The risk to children rises to almost 30% if both parents are diabetic.
Human leukocyte antigen (HLA) class II molecules DR3 and DR4 are associated strongly with type 1 diabetes mellitus. More than 90% of Whites with type 1 diabetes mellitus express one or both of these molecules, compared with 50-60% of the general population.
Patients expressing DR3 are also at risk for developing other autoimmune endocrinopathies and celiac disease. These patients are more likely to develop diabetes at a later age, to have positive islet cell antibodies, and to appear to have a longer period of residual islet cell function.
Patients expressing DR4 are usually younger at diagnosis and more likely to have positive insulin antibodies, yet they are unlikely to have other autoimmune endocrinopathies. The expression of both DR3 and DR4 carries the greatest risk of type 1 diabetes mellitus; these patients have characteristics of both the DR3 and DR4 groups.
Environmental factors
Environmental factors are important because even identical twins have only a 30-60% concordance for type 1 diabetes mellitus and because incidence rates vary in genetically similar populations under different living conditions. [25] No single factor has been identified, but infections and diet are considered the two most likely environmental candidates.
Viral infections may be the most important environmental factor in the development of type 1 diabetes mellitus, [26] probably by initiating or modifying an autoimmune process. Instances have been reported of a direct toxic effect of infection in congenital rubella. One survey suggests that enteroviral infection during pregnancy carries an increased risk of type 1 diabetes mellitus in the offspring. Paradoxically, type 1 diabetes mellitus incidence is higher in areas where the overall burden of infectious disease is lower.
A study from the US Centers for Disease Control and Prevention (CDC) indicates that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), increases the likelihood of diabetes developing in children under age 18 years, more than 30 days post infection. The investigators, using two US health claims databases, reported that pediatric patients with COVID-19 in the HealthVerity database were 31% more likely than other youths to receive a new diabetes diagnosis, while those in the IQVIA database were 166% more likely.
The study could not specify the type or types of diabetes specifically related to COVID-19, with the report saying that the disease could be causing both type 1 and type 2 diabetes but through differing mechanisms. The researchers suggested, however, that COVID-19 may induce diabetes by directly attacking pancreatic cells that express ACE2 receptors, that it may give rise to diabetes “through stress hyperglycemia resulting from the cytokine storm and alterations in glucose metabolism caused by infection,” or that COVID-19 may cause diabetes via the conversion of prediabetes to diabetes. Whether the diabetes is transient or chronic was also unknown. [27, 28]
A study by Kendall et al found that compared with pediatric subjects with a non–SARS-CoV-2 respiratory infection, the proportion of children who were diagnosed with new-onset type 1 diabetes mellitus within 6 months after a SARS-CoV-2 infection was 72% greater. According to the investigators, who looked at patients aged 18 years or younger, the rate of new-onset type 1 diabetes mellitus among the two groups was 0.025% and 0.043%, respectively, at 6 months. [29]
However, a study by Cromer et al looked at adult patients with newly diagnosed diabetes mellitus at the time of hospital admission for COVID-19, finding that a number of them subsequently regressed to a state of normoglycemia or prediabetes. The investigators reported that out of 64 survivors in the study with newly diagnosed diabetes (62 of whom had type 2 diabetes), 26 (40.6%) were known to undergo such regression (median 323-day follow-up). [30]
Moreover, a German study, by Stahl-Pehe et al, found no positive correlation between the incidence of COVID-19 during the pandemic and that of type 1 diabetes in persons under age 20 years. The report looked at the spatiotemporal relationship between waves of COVID-19 cases during the pandemic and the incidence of pediatric type 1 diabetes and accounted “for time lags of up to 12 months between the monthly COVID-19 incidence data and the” incidence data for type 1 diabetes. [31]
Dietary factors are also relevant. Breastfed infants have a lower risk for type 1 diabetes, and a direct relationship is observed between per capita cow's milk consumption and the incidence of diabetes. Some cow's milk proteins (eg, bovine serum albumin) have antigenic similarities to an islet cell antigen.
Nitrosamines, chemicals found in smoked foods and some water supplies, are known to cause type 1 diabetes mellitus in animal models; however, no definite link has been made with humans.
The known association of increasing incidence of type 1 diabetes mellitus with distance from the equator may now have an explanation. Reduced exposure to ultraviolet (UV) light and lower vitamin D levels, both of which are more likely found in the higher latitudes, are associated with an increased risk of type 1 diabetes mellitus. [32]
Chemical causes
Streptozotocin and the rodenticide RH-787 selectively damage islet cells and can cause type 1 diabetes mellitus.
Other causes
Additional factors in the development of type 1 diabetes mellitus include the following:
-
Congenital absence of the pancreas or islet cells
-
Pancreatectomy
-
Pancreatic damage (ie, cystic fibrosis, chronic pancreatitis, thalassemia major, hemochromatosis, hemolytic uremic syndrome)
-
Wolfram syndrome (diabetes insipidus, diabetes mellitus, optic atrophy, deafness [DIDMOAD])
-
Chromosomal disorders such as Down syndrome, Turner syndrome, Klinefelter syndrome, or Prader-Willi syndrome
Epidemiology
Occurrence in the United States
The overall annual incidence of diabetes mellitus is about 24.3 cases per 100,000 person-years. Although most new pediatric diabetes cases are type 1, increasing numbers of children are being diagnosed with type 2 diabetes mellitus, especially among minority groups. [33]
A study by Mayer-Davis et al indicated that between 2002 and 2012, the incidence of type 1 and type 2 diabetes mellitus saw a significant rise among youths in the United States. According to the report, after the figures were adjusted for age, sex, and race or ethnic group, the incidence of type 1 (in patients aged 0-19 years) and type 2 diabetes mellitus (in patients aged 10-19 years) during this period underwent a relative annual increase of 1.8% and 4.8%, respectively. The greatest increases occurred among minority youths. [34]
International occurrence
Type 1 diabetes mellitus has wide geographic variation in incidence and prevalence. [35] Annual incidence varies from 0.61 cases per 100,000 population in China to 41.4 cases per 100,000 population in Finland. Substantial variations are observed between nearby countries with differing lifestyles, such as Estonia and Finland, and between genetically similar populations, such as those in Iceland and Norway.
Also striking are the differences in incidence between mainland Italy (8.4 cases per 100,000 population) and the Island of Sardinia (36.9 cases per 100,000 population). These variations strongly support the importance of environmental factors in the development of type 1 diabetes mellitus. Most countries report that incidence rates have at least doubled in the last 20 years. Incidence appears to increase with distance from the equator. [36]
Race-related demographics
Different environmental effects on type 1 diabetes mellitus development complicate the influence of race, but racial differences are evident. Whites have the highest reported incidence, whereas Chinese individuals have the lowest. Type 1 diabetes mellitus is 1.5 times more likely to develop in American Whites than in American Blacks or Hispanics.
Age-related demographics
Type 1 diabetes mellitus can occur at any age, but incidence rates generally increase with age until midpuberty and then decline. [37] Onset in the first year of life, although unusual, can occur. Type 1 diabetes mellitus must be considered in any infant or toddler, because these children have the greatest risk for mortality if diagnosis is delayed. Because diabetes is easily missed in an infant or preschool-aged child, if in doubt, check urine and finger-stick blood glucose. Symptoms in infants and toddlers may include the following:
-
Severe or recurrent candidal diaper/napkin rash
-
Unexplained malaise
-
Poor weight gain or weight loss
-
Increased thirst
-
Increased wet diapers/urination
-
Vomiting and dehydration
In areas with high prevalence rates, a bimodal variation of incidence has been reported that shows a definite peak in early childhood (ie, ages 4-6 y) and a second, much greater peak of incidence during early puberty (ie, ages 10-14 y). [38]
Prognosis
Apart from severe DKA or hypoglycemia, type 1 diabetes mellitus has little immediate morbidity. The risk of complications relates to diabetic control. With good management, patients can expect to lead full, normal, and healthy lives. Nevertheless, the average life expectancy of a child diagnosed with type 1 diabetes mellitus has been variously suggested to be reduced by 13-19 years, compared with their nondiabetic peers. [39]
Morbidity and mortality
Information on mortality rates for type 1 diabetes mellitus is difficult to ascertain without complete national registers of childhood diabetes, although age-specific mortality is probably double that of the general population. [40, 41] Children aged 1-4 years are particularly at risk and may die due to DKA at the time of diagnosis. Adolescents are also a high-risk group. Most deaths result from delayed diagnosis or neglected treatment and subsequent cerebral edema during treatment for DKA, although untreated hypoglycemia also causes some deaths. Unexplained death during sleep may also occur and appears more likely to affect young males. [42]
A population-based, nationwide cohort study in Finland examined the short -and long-term time trends in mortality among patients with early onset and late-onset type 1 diabetes. The results suggest that in those with early onset type 1 diabetes (age 0-14 y), survival has improved over time. Survival of those with late-onset type 1 diabetes (15-29 y) has deteriorated since the 1980s, and the ratio of deaths caused by acute complications has increased in this group. Overall, alcohol was noted as an important cause of death in patients with type 1 diabetes; women had higher standardized mortality ratios than did men in both groups. [43]
The complications of type 1 diabetes mellitus can be divided into three major categories: acute complications, long-term complications, and complications caused by associated autoimmune diseases.
Acute complications, which include hypoglycemia, hyperglycemia, and DKA, reflect the difficulties of maintaining a balance between insulin therapy, dietary intake, and exercise.
Long-term complications arise from the damaging effects of prolonged hyperglycemia and other metabolic consequences of insulin deficiency on various tissues. Although long-term complications are rare in childhood, maintaining good control of diabetes is important to prevent complications from developing in later life. [44] The likelihood of developing complications appears to depend on the interaction of factors such as metabolic control, genetic susceptibility, lifestyle (eg, smoking, diet, exercise), pubertal status, and gender. [45, 46] Long-term complications include the following:
-
Retinopathy
-
Cataracts
-
Gastroparesis
-
Progressive renal failure
-
Early coronary artery disease
-
Peripheral vascular disease
-
Peripheral and autonomic neuropathy
-
Increased risk of infection
Associated autoimmune diseases are common in type 1 diabetes mellitus, particularly in children who have HLA-DR3. Some conditions may precede the development of diabetes, and others may develop later. As many as 20% of children with diabetes have thyroid autoantibodies. [47]
Type 1 diabetes in pediatric patients has been linked to changes in cognition and brain structure, with a study by Siller et al finding lower volume in the left temporal-parietal-occipital cortex in young patients with type 1 diabetes than in controls. The study also indicated that in pediatric patients, higher severity of type 1 diabetes presentation correlates with greater structural differences in the brain at about 3 months following diagnosis.
The investigators found that among study patients with type 1 diabetes, an association existed between the presence of diabetic ketoacidosis at presentation and reduced radial, axial, and mean diffusivity in the major white matter tracts on magnetic resonance imaging (MRI). In those with higher glycated hemoglobin (HbA1c) levels, hippocampal, thalamic, and cerebellar white matter volumes were lower, as was right posterior parietal cortical thickness, while right occipital cortical thickness was greater. Patients in the study were aged 7-17 years. [48]
A study by Dabelea et al found that in teenagers and young adults in whom diabetes mellitus had been diagnosed during childhood or adolescence, diabetes-related complications and comorbidities—including diabetic kidney disease, retinopathy, and peripheral neuropathy (but not arterial stiffness or hypertension)—were more prevalent in those with type 2 diabetes than in those with type 1 disease. [49]
COVID-19
A study indicated that children with type 1 diabetes mellitus who have an HbA1c level of 9% or above are at greater risk for mortality, intubation, and sepsis due to COVID-19 than are children without type 1 diabetes. However, the report also found evidence that such risk is not greater in children with an HbA1c level at or below 7%. The investigators found the COVID-19 mortality rates in children without type 1 diabetes, those with type 1 diabetes, and those with type 1 diabetes with an HbA1c of 7% or lower to be 0.047%, 0.328%, and 0%, respectively. [50]
Patient Education
Education is a continuing process involving the child, family, and all members of the diabetes team. [51, 52] The following strategies may be used:
-
Formal education sessions in a clinic setting
-
Opportunistic teaching at clinics or at home in response to crises or difficulties such as acute illness
-
Therapeutic camping or other organized events
-
Patient-organized meetings
-
Education of school officials/caretakers on the basics of diabetes management; see the ADA's website for videos - https://diabetes.org/advocacy/safe-at-school-state-laws/training-resources-school-staff
Diabetes-related organizations and patient resources include the following:
-
Possible mechanism for development of type 1 diabetes.
-
The effects of insulin deficiency.
-
Representation of activity profile of some available insulins.