Overview
The skin covers the entire external surface of the human body and is the principal site of interaction with the surrounding world. It serves as a protective barrier that prevents internal tissues from exposure to trauma, ultraviolet (UV) radiation, temperature extremes, toxins, and bacteria. Other important functions include sensory perception, immunologic surveillance, thermoregulation, and control of insensible fluid loss.
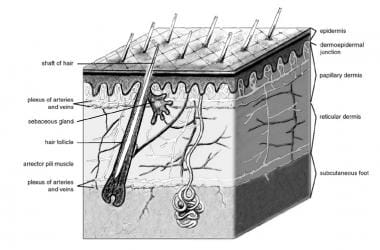
The integument consists of three mutually dependent layers, the epidermis, dermis and hypodermis. [1, 2] The hypodermis is rich in fatty tissue known as the panniculus adiposus, which may be arranged in clusters. The epidermis is derived primarily from surface ectoderm but is colonized by pigment-containing melanocytes of neural crest origin, antigen-processing Langerhans cells (LCs) of bone marrow origin, and pressure-sensing Merkel cells of neural crest origin. It contains keratinocytes organized into layers: the basal layer (stratum basale), spinous layer (stratum spinosum), granular layer (stratum granulosum), stratum lucidum (in acral skin of palms and soles), and the outermost layer (stratum corneum). These layers facilitate keratinization and maintain the epidermal water barrier through lipid envelopes and protein cross-linking processes. [3, 4]
The dermis is derived primarily from mesoderm. It is subdivided into the papillary dermis (loose connective tissue) and reticular dermis (dense connective tissue) [3, 4] and contains collagen, elastic fibers, blood vessels, sensory structures, and fibroblasts. [5]
The hypodermis anchors the dermis to underlying structures and provides insulation and cushioning through adipose tissue. [4]
See the image below.
During the fourth week of embryologic development, the single cell thick ectoderm and underlying mesoderm begin to proliferate and differentiate. The specialized structures formed by the skin, including teeth, hair, hair follicles, fingernails, toenails, sebaceous glands, sweat glands, apocrine glands, and mammary glands also begin to appear during this period in development. Teeth, hair, and hair follicles are formed by the epidermis and dermis in concert, while fingernails and toenails are formed by the epidermis alone. Hair follicles, sebaceous glands, sweat glands, apocrine glands, and mammary glands are considered epidermal glands or epidermal appendages, because they develop as downgrowth or diverticula of the epidermis into the dermis. [5, 6]
The definitive multi-layered skin is present at birth, but skin is a dynamic organ that undergoes continuous changes throughout life as outer layers are shed and replaced by inner layers. Skin also varies in thickness among anatomic location, sex, and age of the individual. This varying thickness primarily represents a difference in dermal thickness, as epidermal thickness is rather constant throughout life and from one anatomic location to another. Skin is thickest on the palms and soles of the feet (1.5 mm thick), while the thinnest skin is found on the eyelids and in the postauricular region (0.05 mm thick).
Male skin is characteristically thicker than female skin in all anatomic locations. Children have relatively thin skin, which progressively thickens until the fourth or fifth decade of life when it begins to thin. This thinning is also primarily a dermal change, with loss of elastic fibers, epithelial appendages, and ground substance. [7]
Epidermis
The epidermis contains no blood vessels and is entirely dependent on the underlying dermis for nutrient delivery and waste disposal via diffusion through the dermoepidermal junction (DEJ). The epidermis is a stratified, squamous epithelium that consists primarily of keratinocytes in progressive stages of differentiation from deeper to more superficial layers. The named layers of the epidermis include the stratum germinativum, stratum spinosum, stratum granulosum, and stratum corneum.
Stratum basale (or stratum germinativum): This basal layer is composed of a single row of cuboidal to columnar keratinocytes that are mitotically active, ensuring continuous regeneration of the epidermis. It also contains melanocytes (responsible for melanin production), Merkel cells (mechanoreceptors for light touch), and LCs (immune cells). Hemidesmosomes anchor the basal keratinocytes to the basement membrane, which separates the epidermis from the dermis. [4]
Stratum spinosum: Known as the "prickle cell layer," this layer consists of several layers of keratinocytes interconnected by desmosomes, providing mechanical strength. Keratinocytes in this layer contain prominent bundles of cytokeratin filaments, primarily cytokeratins 1 (K1) and 10 (K10). [4]
Stratum granulosum: This layer contains 3-5 layers of flattened keratinocytes rich in keratohyalin granules (profilaggrin and loricrin) and lamellar bodies filled with lipids. These components contribute to the formation of the epidermal water barrier. [4]
Stratum lucidum (clear layer): This layer is found in areas such as the palms and soles. The cells in this layer contain compacted keratin filaments. It is a thin, translucent layer consisting of dead, flattened keratinocytes that often contain nuclear debris. [4]
Stratum corneum: The outermost layer comprises enucleated corneocytes embedded in a lipid matrix. This layer provides a robust barrier against water loss and environmental damage through its "brick-and-mortar" structure — corneocytes ("bricks") surrounded by lipid ("mortar"). [4]
Keratinocytes
The stratum germinativum, or the basal layer, is immediately superficial to the DEJ. This single cell layer of keratinocytes is attached to the basement membrane via hemidesmosomes. Hemidesmosomes provide mechanical stability and facilitate signaling between the epidermis and dermis. [1]
Keratinocytes in the basal layer are mitotically active, serving as progenitor cells for all other keratinocytes in the epidermis. As they divide and differentiate, keratinocytes migrate upward through successive layers of the epidermis. In the stratum spinosum, keratinocytes begin to synthesize keratins and are interconnected by desmosomes, forming an important structural network. In the stratum granulosum, keratinocytes accumulate keratohyalin granules and lamellar bodies, which contribute to the epidermal barrier by forming a lipid matrix. [8]
Once they reach the stratum corneum, they are fully differentiated keratinocytes devoid of nuclei and are subsequently shed in the process of epidermal turnover. Cells of the stratum corneum are the largest and most abundant of the epidermis. Upon reaching the stratum corneum, keratinocytes undergo terminal differentiation into corneocytes — anucleate, flattened cells embedded in a lipid-rich matrix. The corneocytes are bound by corneodesmosomes and form a durable, waterproof barrier that protects against environmental insults such as pathogens, UV radiation, and mechanical stress. The thickness of the stratum corneum varies across anatomical sites, ranging from 15-20 layers in thin skin to over 100 layers in regions such as the palms and soles. [8, 9]
The process of epidermal turnover, from basal cell division to desquamation at the surface, takes approximately 28-40 days under normal conditions but may vary with age or underlying diseases. This continuous renewal ensures maintenance of the skin's protective functions while allowing adaptation to external stimuli. [10]
Melanocytes
Melanocytes, derived from neural crest cells, primarily function to produce a pigment, melanin, which absorbs radiant energy from the sun and protects the skin from the harmful effects of UV radiation. Melanin exists in two primary forms: eumelanin (dark brown or black) and pheomelanin (red or yellow), with eumelanin being more effective in photoprotection due to its superior UV-absorbing capacity. [11] Melanocytes are found in the basal layer of the epidermis as well as in hair follicles, the retina, uveal tract, and leptomeninges. These cells are the sites of origin of malignant melanoma.
Melanin is synthesized within specialized organelles called melanosomes through a series of enzymatic reactions involving tyrosinase, tyrosinase-related protein 1, and dopachrome tautomerase. The melanosomes mature through four distinct stages, culminating in fully melanized organelles. These are transported along melanocyte dendrites and transferred to keratinocytes via mechanisms such as phagocytosis, exocytosis-endocytosis, and cytophagocytosis. Within keratinocytes, melanosomes form supranuclear caps that protect DNA from UV-induced damage. [12]
In areas exposed to the sun, the ratio of melanocytes to keratinocytes is approximately 1:4. In areas not exposed to solar radiation, the ratio may be as small as 1:30. Absolute numbers of melanosomes are the same among the sexes and various races. Differing pigmentation among individuals is related to melanosome size rather than cell number. Sun exposure, melanocyte-stimulating hormone, adrenocorticotropic hormone, estrogens, and progesterone stimulate melanin production.
With aging, melanocyte density declines at an estimated rate of 6-8% per decade in both sun-exposed and protected skin. Since these cells are of neural crest origin, they have no ability to reproduce. This reduction contributes to hypopigmentation observed in elderly individuals. Additionally, senescent melanocytes accumulate markers such as p16INK4a and exhibit altered functionality. These cells can influence neighboring keratinocytes and fibroblasts through paracrine signaling, contributing to age-related pigmentation disorders such as solar lentigines. [13]
Melanocytes originate from neural crest cells during embryogenesis. These progenitors migrate along a dorsolateral pathway to colonize the epidermis and other sites. In adulthood, melanocyte stem cells located in hair follicle bulges serve as a reservoir for replenishing melanocytes during homeostasis or after injury. Schwann cell precursors have also been identified as a secondary source of melanocytes under specific conditions such as wound repair. [14]
Langerhans Cells
LCs are specialized dendritic cells residing predominantly in the stratum spinosum of the epidermis. [15] LCs originate from the bone marrow and are found in the basal, spinous, and granular layers of the epidermis. They serve as antigen-presenting cells. They are capable of ingesting foreign antigens, processing them into small peptide fragments, binding them with major histocompatibility complexes, and subsequently presenting them to lymphocytes for activation of the immune system. An example of activation of this component of the immune system is contact hypersensitivity. Studies suggest that LCs may also play a role in maintaining immune tolerance. [16]
Merkel Cells
The origin of Merkel cells has been a topic of debate. While initially thought to derive from neural crest cells, evidence supports an epithelial origin. Study models have demonstrated that Merkel cells arise from epidermal progenitors expressing the transcription factor Atoh1 during development. This epithelial origin is further supported by their expression of cytokeratins, such as CK20, which are characteristic of epithelial cells. [17] They are found on the volar aspect of digits, in nail beds, on the genitalia, and in other areas of the skin. These cells are specialized in the perception of light touch. [5, 7]
Dermis
The primary function of the dermis is to sustain and support the epidermis. It is a complex connective tissue layer of mesenchymal origin, situated between the epidermis and subcutaneous tissue. The dermis is primarily composed of collagen and elastic fibers interspersed within an amorphous extracellular ground substance made up of glycosaminoglycans (e.g., hyaluronic acid), proteoglycans, and glycoproteins. This structural matrix provides mechanical strength, elasticity, and resilience to the skin. [18] The dermis is composed of two layers, the more superficial papillary dermis and the deeper reticular dermis.
The papillary dermis is thinner, consisting of loose connective tissue containing capillaries, elastic fibers, reticular fibers, and some collagen. Dermal papillae, finger-like projections from this layer, interdigitate with epidermal ridges, enhancing the connection between the dermis and epidermis. The papillary dermis is highly vascularized and contains sensory nerve endings that contribute to tactile sensation. [1]
The reticular dermis consists of a thicker layer of dense connective tissue containing larger blood vessels, closely interlaced elastic fibers, and coarse bundles of collagen fibers arranged in layers parallel to the surface. The reticular layer also contains fibroblasts, mast cells, nerve endings, lymphatics, and epidermal appendages.
Surrounding the components of the dermis is the gel-like ground substance, composed of mucopolysaccharides (primarily hyaluronic acid), chondroitin sulfates, and glycoproteins. This matrix facilitates hydration, nutrient diffusion, and mechanical support for cellular components. [1] The deep surface of the dermis is highly irregular and borders the subcutaneous layer, the panniculus adiposus, which additionally cushions the skin.
Fibroblasts
The fibroblast is the major cell type of the dermis. These cells produce and secrete procollagen and elastic fibers. Procollagen is terminally cleaved by proteolytic enzymes into collagen that aggregates and becomes cross-linked. This collagen aggregates into fibrils that are cross-linked by lysyl oxidase, a copper-dependent enzyme, to enhance tensile strength and resistance to shear and other mechanical forces. [19] Collagen makes up 70% of the weight of the dermis, primarily type I (85% of the total collagen) and type III (15% of the total collagen).
Elastic fibers, composed of elastin and fibrillin microfibrils, [1] constitute less than 1% of the weight of the dermis, but they play an enormous functional role by resisting deformational forces and returning the skin to its resting shape. [5]
Studies highlight fibroblast heterogeneity within the dermis. Papillary fibroblasts produce non-fibrillar collagens (e.g., type IV) and proteoglycans such as decorin, contributing to scar-free wound healing. Reticular fibroblasts predominantly synthesize fibrillar collagens (types I and III) and are involved in fibrotic processes. This functional distinction is essential for understanding their roles in skin repair, aging, and disease. [20, 21]
Fibroblasts also play a pivotal role in wound healing by migrating to injury sites, proliferating, and producing extracellular matrix components. They can differentiate into myofibroblasts under the influence of transforming growth factor-beta, which facilitates wound contraction through actin-myosin complexes. [22]
Dermoepidermal Junction
The DEJ is an undulating basement membrane that adheres the epidermis to the dermis. It is composed of two layers, the lamina lucida and lamina densa. The lamina lucida is thinner and lies directly beneath the basal layer of epidermal keratinocytes. It primarily contains transmembrane proteins such as laminin-332 (formerly laminin-5) and integrins, which mediate adhesion between basal keratinocytes and the basement membrane. [23]
The thicker lamina densa is in direct contact with the underlying dermis. It is composed predominantly of type IV collagen. It interacts with anchoring fibrils made of type VII collagen, which extend into the dermis to secure the connection between the epidermis and dermis. [24]
These structures are the target of immunologic injury in disorders such as bullous pemphigoid and epidermolysis bullosa.
Dermal papillae from the papillary dermis contain a plexus of capillaries and lymphatics oriented perpendicular to the skin surface. These finger-like projections are surrounded by similar projections of the epidermis. This highly irregular junction greatly increases the surface area over which oxygen, nutrients, and waste products are exchanged between the dermis and the avascular epidermis. [5]
Epidermal Appendages
Epidermal appendages are intradermal epithelial structures lined with epithelial cells with the potential for division and differentiation. These are important as a source of epithelial cells, which accomplish reepithelialization should the overlying epidermis be removed or destroyed in situations such as partial thickness burns, abrasions, or split-thickness skin graft harvesting.
Epidermal appendages include the following:
-
Sebaceous glands
-
Sweat glands
-
Apocrine glands
-
Mammary glands
-
Hair follicles
They often are found deep within the dermis and, in the face, may even lie in the subcutaneous fat beneath the dermis. This accounts for the remarkable ability of the face to re-epithelialize even the deepest cutaneous wounds. [5]
Studies have highlighted the significant role of epidermal appendages in wound healing. For instance, hair follicles and sebaceous glands contain progenitor cells that actively participate in reepithelialization. These progenitor cells migrate to the wound site, differentiate into keratinocytes, and contribute to the formation of new epidermis, thereby enhancing the healing process. [25]
Eccrine sweat glands have been identified as major contributors to reepithelialization. Research indicates that during wound healing, epithelial outgrowths from sweat glands expand and merge, reconstituting the new epidermis. This finding shows the importance of sweat glands in skin regeneration and repair. [25, 26]
Sebaceous Glands
Sebaceous glands, or holocrine glands, are found over the entire surface of the body except the palms, soles, and dorsum of the feet. They are largest and most concentrated on the face and scalp, where they are the sites of origin of acne. The normal function of sebaceous glands is to produce and secrete sebum, a group of complex oils that include triglycerides and fatty acid breakdown products, wax esters, squalene, cholesterol esters, and cholesterol. Sebum plays a crucial role in maintaining skin homeostasis by lubricating the skin, protecting against friction, and providing a barrier against moisture and microbial invasion. [27]
Research highlights additional roles for sebaceous glands in skin homeostasis, including contributions to innate immunity and antioxidative defense mechanisms. Sebaceous glands possess stem cell populations that enable self-renewal and regeneration following injury. They secrete antimicrobial lipids and maintain the acid mantle, which inhibits the growth of pathogenic microorganisms. [28]
Sweat Glands
Sweat glands, or eccrine glands, are found over the entire surface of the body except the vermillion border of the lips, the external ear canal, the nail beds, the labia minora, and the glans penis and the inner aspect of the prepuce. They are most concentrated in the palms, soles, and the axillae.
Each gland consists of a coiled secretory intradermal portion that connects to the epidermis via a relatively straight distal duct. The normal function of the sweat gland is to produce sweat, which cools the body by evaporation. The thermoregulatory center in the hypothalamus controls sweat gland activity through sympathetic nerve fibers that innervate the sweat glands. Sweat excretion is triggered when core body temperature reaches or exceeds a set point.
Apocrine and Mammary Glands
Apocrine glands are similar in structure, but not identical, to eccrine glands. They are found in the axillae, in the anogenital region, and, as modified glands, in the external ear canal (ceruminous glands), the eyelid (Moll's glands), and the breast (mammary glands). They produce odor and do not function prior to puberty, which means they probably serve a vestigial function. The mammary gland is considered a modified and highly specialized type of apocrine gland.
Hair Follicles
Hair follicles are complex structures formed by the epidermis and dermis (see the image below). They are found over the entire surface of the body except the soles of the feet, palms, glans penis, clitoris, labia minora, mucocutaneous junction, and portions of the fingers and toes. Sebaceous glands often open into the hair follicle rather than directly onto the skin surface, and the entire complex is termed the pilosebaceous unit. [5, 29, 30]
Hair follicle orientation and shape determines hair texture, where a vertically oriented round follicle will grow straight hair, an obliquely oriented oval follicle with grow wavy, and almost parallel orientation of flat follicles with grow tight curls. These anatomic variations are an important consideration in avoiding alopecia when making incisions in the scalp.
The base of the hair follicle, or hair bulb, lies deep within the dermis and subcutaneous fat and at its base includes the mesenchymal-derived dermal papilla, a neurovascular structure that supplies nutrients to the proliferating matrix and helps form the hair shaft. A band of smooth muscle, the arrector pili, connects the deep portion of the follicle to the superficial dermis. Contraction of this muscle, under control of the sympathetic nervous system, causes the follicle to assume a more vertical orientation. Piloerection of densely populated hair follicles facilitates air trapping for insulation in cold temperatures and makes the animal appear larger in response to emotional stimuli. It is thought to be a vestigial trait in thermoregulation and in the “fight-or-flight” response. [31]
Hair follicles are composed of concentric layers: the hair shaft, comprising keratinized cells organized into the medulla, cortex, and cuticle. The inner root sheath anchors the hair shaft and has three layers: Henle's layer, Huxley's layer, and a cuticle layer. The outer root sheath provides structural support and houses multipotent stem cells critical for follicular regeneration. [32]
Hair growth exhibits a cyclical pattern. The anagen phase is the growth phase, whereas the telogen phase is the resting state. The transition between anagen and telogen is termed the catagen phase. Phases vary in length according to anatomic location, and the length of the anagen phase is proportional to the length of the hair produced. At any one time at an anatomic location, follicles are found in all three phases of hair growth. This is extremely important for laser hair removal, where through the process of selective photothermolysis laser energy targets melanin in the hair shaft and transfers heat to the hair bulb thereby damaging follicular stem cells in the bulge of the outer root sheath and/or the dermal papilla at the base of the follicle. Since the bulb in catagen phase undergoes apoptosis, the hair shaft stops growing and breaks away from the dermal papilla, laser hair removal does not have a sufficient chromophore target in hair outside of anagen phase. [33] This explains why multiple treatments of an area may be necessary to ensure adequate hair removal.
Cutaneous Blood Supply
Cutaneous vessels ultimately arise from underlying named source vessels. Each source vessel supplies a three-dimensional vascular territory from bone to skin termed an angiosome. Adjacent angiosomes have vascular connections via reduced caliber (choke) vessels or similar caliber (true) anastomotic vessels. The cutaneous vessels originate either directly from the source arteries (septocutaneous or fasciocutaneous perforators) or as terminal branches of muscular vessels (musculocutaneous perforators).
During their course to the skin, the cutaneous vessels travel within or adjacent to the connective tissue framework and supply branches to each tissue with which they come into close contact (bone, muscle, fascia, nerve, fat). They emerge from the deep fascia in the vicinity of the intermuscular or intramuscular septa or near tendons and travel toward the skin, where they form extensive subdermal and dermal plexuses. The dermis contains horizontally arranged superficial and deep plexuses, which are interconnected via communicating vessels oriented perpendicular to the skin surface. Cutaneous vessels ultimately anastomose with other cutaneous vessels to form a continuous vascular network within the skin. Clinically, this extensive horizontal network of vessels allows for random skin flap survival. [34, 35, 36]
Studies have further detailed the organization of dermal arteries into discrete arterial units, challenging the traditional concept of a continuous superficial dermal arterial plexus. Research on the cutaneous angiosome of the descending genicular artery has demonstrated that dermal arteries form tree-like ramifications, supplying specific dermal volumes, with limited arterio-arterial anastomoses, particularly in the central regions of an angiosome. [37]
Thermoregulation
In addition to the skin's natural heat conductivity and loss of heat from the evaporation of sweat, convection from cutaneous vessels is a vital component of thermoregulation. Cutaneous blood flow is 10-20 times that required for essential oxygenation and metabolism, and large amounts of heat can be exchanged through the regulation of cutaneous blood flow. The thermoregulatory center in the hypothalamus controls vasoconstriction and vasodilatation of cutaneous vessels through the sympathetic nervous system.
Lymphatics
Skin lymphatics parallel the blood supply and function to conserve plasma proteins and scavenge foreign material, antigenic substances, and bacteria. Blind-ended lymphatic capillaries arise within the interstitial spaces of the dermal papillae. These unvalved, superficial dermal vessels drain into valved deep dermal and subdermal plexuses. These then coalesce to form larger lymphatic channels, which course through numerous filtering lymph nodes on their way to join the venous circulation near the junction of the subclavian and internal jugular veins bilaterally.
Skin lymphatics play a pivotal role in regeneration and immune modulation. Lymphatic vessels interact with skin stem cells, such as those in hair follicles, to coordinate tissue repair processes. Lymphatics actively participate in lipid transport, particularly in reverse cholesterol transport, emphasizing their metabolic importance. [38, 39]
Skin Innervation
Sensory perception is critically important in the avoidance of pressure, mechanical or traumatic forces, and extremes of temperature. Numerous specialized structures are present in the skin to detect various stimuli. As previously mentioned, Merkel cells of the epidermis are mechanoreceptors that detect light touch. Meissner corpuscles detect discriminatory light touch and low frequency vibration. These are found in the dermal papillae and are most concentrated in the fingertips. Pacini corpuscles are found deep within the dermis or even in the subcutaneous tissue and detect pressure.
Pain is transmitted through naked nerve endings located in the basal layer of the epidermis. Krause bulbs detect cold, whereas Ruffini corpuscles detect heat. Heat, cold, and proprioception also are located in the superficial dermis. Cutaneous nerves follow the route of blood vessels to the skin. The area supplied by a single spinal nerve, or a single segment of the spinal cord, is termed a dermatome. Adjacent dermatomes may overlap considerably, which is important to note when performing field blocks with local anesthesia. [5, 40]
Surface Anatomy
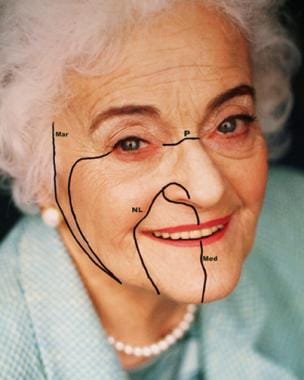
Lines and creases are evident over major and minor joints. Skin contraction produces wrinkles and creases that lie perpendicular to the underlying muscular vector force. Relaxed skin tension lines (RSTL), however, are formed during relaxation and often follow a different direction than age and contracting wrinkles (see the image below). RSTL are created by the natural tension on the skin from underlying structures. [41]
Papillary ridges on the tips of the digits of the hands and feet and the surface of palms and soles are often used for personal identification. These are also known as friction ridges, since they assist in the ability to grasp. They are formed during fetal development and are unique to each individual, including identical twins. This distinct pattern does not change with aging. Stratum mucosum composes the outer surface of the ridges with underlying dermal papillae. Sweat pores are usually located at the top of the ridges. [42]
Skin Phototype
The amount of melanin pigment in the skin determines an individual's skin color (skin phototype). Skin pigment can be inherited genetically or can be acquired through various diseases. Hormonal changes during pregnancy can also vary the amount of pigmentation.
The Fitzpatrick scale is used to classify skin complexion and response to UV exposure (see Table 1 below). This classification is based on a personal history of sunburning and suntanning. [43] This classification is used clinically for the evaluation of facial skin pigmentation before resurfacing procedures and is important for predicting outcomes and adverse effects.
Table 1. The Fitzpatrick Scale (Open Table in a new window)
Skin Type |
Color |
Features |
I |
White or freckled skin |
Always burns, never tans |
II |
White skin |
Burns easily, tans poorly |
III |
Olive skin |
Mild burn, gradually tans |
IV |
Light brown skin |
Burns minimally, tans easily |
V |
Dark brown skin |
Rarely burns, tans easily |
VI |
Black skin |
Never burns, always tans |
This classification is integral for tailoring dermatologic interventions to individual patient needs. Patients with types I and II are at a higher risk of UV-induced damage and require aggressive photoprotection. Individuals with types V and VI are more prone to post-inflammatory hyperpigmentation after procedures or trauma. [44]
Studies have also highlighted the limitations of Fitzpatrick scale in capturing the full spectrum of global skin diversity. Emerging classification systems aim to incorporate additional variables such as genetic ancestry and advanced imaging technologies for more precise assessments of cutaneous phototypes. However, the Fitzpatrick scale remains a cornerstone in clinical practice due to its simplicity and utility. [44]
Anatomy of Aging Skin
Age-associated skin changes include thinning, skin laxity, fragility, and wrinkles. Sun-exposed areas demonstrate additional aging changes, including dyspigmentation, premature wrinkling, telangiectasia, and actinic elastosis.
Cutaneous aging is characterized by intrinsic and extrinsic processes. Intrinsic, or chronologic, aging is a genetically determined and inevitable process in skin, including photoprotected skin. Intrinsic aging naturally occurs and is exacerbated by extrinsic aging, which is environmentally induced.
Aging at the cellular level is thought to be related to cellular senescence, specifically, the shortening of telomeres (the terminal portions of chromosomes) with each cell cycle. Telomere shortening ultimately results in cell-cycle arrest or apoptosis once a critical length is reached.
Preventable environmental factors that amplify intrinsic aging include exposure to ultraviolet radiation and air pollution, nutritional intake, and consumption of alcohol and tobacco products. Long-term UVA radiation exposure accelerates intrinsic aging via the formation of reactive oxygen species (ROS). ROS lead to inflammatory cytokines and the up-regulation of matrix metalloproteinases, which result in the breakdown of collagen. UVB radiation can also contribute to this aging process by causing direct deoxyribonucleic acid mutations.
Histopathologically, photoaging is manifest as flattening of the DEJ, resulting in decreased nutrient transfer between the layers, heliodermatitis or chronic inflammation, elongated and collapsed fibroblasts, disorganized collagen fibrils with overall decrease in collagen levels, and the accumulation of abnormal elastin-containing material termed solar elastosis. [45, 46]
-
Anatomy of the skin.
-
Four main facial lines show the direction of relaxed skin tension lines.
-
Anatomy of hair follicle.
-
Anatomy of Hair Follicle.