Practice Essentials
The pituitary gland is the master gland of the body because it controls most of the body's endocrine functions by means of the hypothalamic-pituitary axis (see the images below). The anterior lobe of the pituitary gland secretes 6 hormones: thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), leuteinizing hormone (LH), growth hormone (GH), and prolactin (PRL). The posterior pituitary gland secretes vasopressin and oxytocin. For pituitary adenoma imaging, CT and MRI have largely replaced plain radiography because conventional radiography is poor for delineating soft tissues. [1, 2, 3, 4, 5, 6, 7, 8, 9]
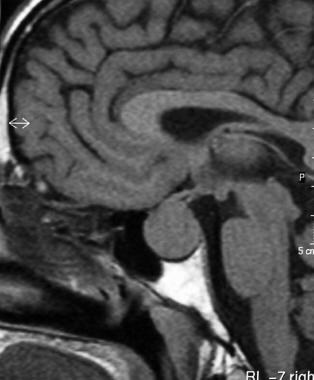
 T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
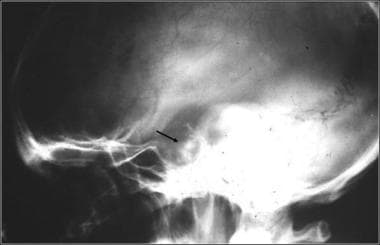 Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
Pituitary adenomas occur primarily in the adenohypophysis and represent 10-25% of all intracranial neoplasms; estimated prevalence in the general population is around 17%. [10, 11, 12] The World Health Organization (WHO) classifies pituitary adenomas as typical adenomas, atypical adenomas, and pituitary carcinomas and encourages taking into account tumor invasion (evaluated by MRI and/or histology) and histopathologic parameters. [13]
Pituitary adenomas are almost always benign with no malignant potential. In general, pituitary lesions can be subdivided into nonsecretory and secretory tumors of the pituitary gland, other intrasellar tumors, and parasellar tumors. The last group occurs in the vicinity of the sellar turcica and can mimic the pituitary tumors in terms of the symptoms they cause. Nonsecretory pituitary tumors are called null-cell tumors.
Computed Tomography
In the ideal situation, CT of the pituitary gland is performed with multidetector-row CT. [24] Volumetric (64-section) CT scanners produce coronal reformations with a resolution near that of direct coronal acquisitions. Single-channel axial and helical CT of the pituitary gland is best performed in the direct coronal plane by maximally extending the patient's neck while the patient is either supine or prone. This method allows for the demonstration of pituitary abnormalities with radiation dose to the lens that is lower than that possible with axial imaging.
As an alternative, pituitary CT can be performed in the axial plane with thin (1-mm) contiguous sections after the intravenous administration of 100 mL of contrast medium. Exposure parameters are approximately 120 kV, 200 mA, 2-second scanning time, and a soft tissue algorithm. Images are reformatted in the coronal and sagittal planes.
Microadenomas are small, round tumors embedded in the parenchyma of the pituitary gland. Microadenomas uncomplicated by hemorrhage or cyst formation are typically isoattenuating or hypoattenuating relative to the adjacent normal pituitary gland. They may not be visible on nonenhanced CT scans. Enhancement after the administration of contrast material occurs but is delayed compared with the immediate, intense enhancement of the normal pituitary gland. This effect is due to the absence of a blood-brain barrier while the adenoma remains hypoattenuating. Therefore, about two thirds of microadenomas typically appear hypoattenuating on dynamic, rapid-sequence, contrast-enhanced CT scans, and one third show early enhancement.
Macroadenomas have variable appearances. Most are isoattenuating relative to the cortex on nonenhanced CT scans and show moderate enhancement on enhanced scans. Calcification is rare (1-8%). Necrosis, cyst formation, and hemorrhage may result in lesions of mixed attenuation. CT also shows bony changes and the mass lesion, with expansion of the sella turcica with or without erosion or depression of the sellar floor toward the sphenoid sinus. Secreting adenomas protrude or expand into the cavernous sinus more often than do nonsecreting macroadenomas. [25] In summary, microadenomas are hypoattenuating relative to the normal pituitary on dynamic enhanced CT scans, and macroadenomas are isoattenuating on nonenhanced CT scans and intensely enhancing on enhanced scans.
CTA is extremely useful in preoperative planning for macroadenomas. The relationship of the tumor to the anterior cerebral arteries and optic nerve are critical to the surgeon. Image guidance protocols for thin-section CT are also useful during surgery itself.
Although MRI is the imaging modality of choice to study pituitary adenomas, CT still has a role because many patients cannot undergo MRI. CT is also useful to depict calcification, which may influence the differential diagnosis. CT may contribute to preoperative planning, particularly in regard to pneumatization and the anatomy of the sphenoid sinus. The drawback of CT is that its ability to show the soft tissue is suboptimal compared with that of MRI. In addition, contrast medium is used to enhance images, and patients are exposed to radiation.
A study of 28 patients with surgically confirmed functional pituitary microadenomas showed that dynamic contrast-enhanced multisection CT combined with image reconstruction holds promise in detecting MRI-occult pituitary microadenomas. In this study, 15 cases were correctly diagnosed by MRI, while dynamic contrast-enhanced multisection CT correctly diagnosed 26 cases. The accuracy of localization was markedly better for adrenocorticotropic hormone-secreting microadenomas, increasing from 32% on MRI to 85% by dynamic contrast-enhanced multisection CT. [26]
The sensitivity of conventional CT in detecting pituitary microadenomas is 17-22%. The accuracy of modern scanners has not yet been evaluated.
Magnetic Resonance Imaging
In children, the height of normal pituitary gland can be evaluated as a function of age. Pituitary gland heights (PGHs) have been measured on strictly sagittal T1-weighted images obtained with 3- to 7-mm-thick sections. The measurement is taken at the greatest height, which is usually the midpoint. PGH physiologically enlarges at birth, at puberty (6-7 mm), during pregnancy (< 10 mm), and after birth (< 12 mm).
Sex-related differences in the PGH are observed. In the age group of 10-69 years, pituitary height is greater in female individuals then in male individuals. In pediatric patients aged 0-9 years, both sexes have minimal pituitary height. Maximal height is observed in the 10- to 19-year age group. The height gradually decreases with age after 20 years. In a study by Suzuki et al, no females had a PGH of 9 mm or more, and no males had a PGH of 8 mm of more. [27]
On MRI, the normal anterior pituitary gland and its stalk return uniform isointensity relative to gray matter. These structures also show intense enhancement after the administration of contrast agent. The gland may be hyperintense in neonates and in pregnant women. The normal posterior pituitary appears bright on T1-weighted MRIs. In about 10% of individuals, MRIs show focal pituitary abnormalities, which are thought to represent protein molecules in the gland.
On T2- and nonenhanced T1-weighted MRIs, the normal anterior lobe of the pituitary gland is isointense relative to the gray matter of the brain. The posterior lobe is hyperintense on T1-weighted images. The high proteinaceous content of the neurosecretory vesicles in the posterior pituitary lobe partly accounts for this appearance.
Nonneoplastic cysts are seen in 20% of autopsies and may represent a normal variant or glandular degeneration. In women of childbearing age, the pituitary gland varies substantially in size over the course of the menstrual cycle. In these women, the normal gland may have a convex superior surface, and it may appear to bulge out of the sella turcica.
(The pituitary, cysts, adenomas, and metastases on MRI are displayed in the images below.)
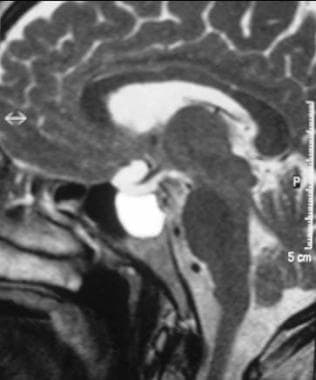 Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
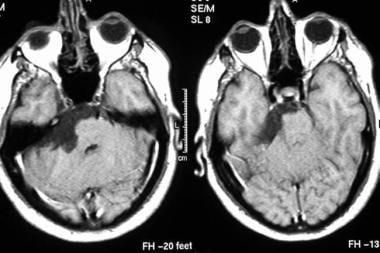 Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
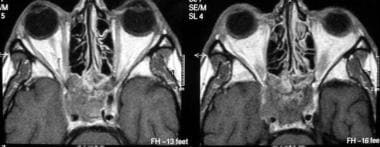 Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
Microadenomas
In the evaluation of pituitary microadenomas, the key issues are the presence or absence of an adenoma, the location of the adenoma, and its invasive effects on the adjacent structures. High-resolution, thin-section MRI of the pituitary gland in the coronal and sagittal planes is the imaging modality of choice.
Pituitary microadenomas are usually slightly hypointense to isointense to the gray matter on T1-weighted images and range from isointense to hyperintense on T2-weighted images unless they contain intratumoral hemorrhage or cystic changes. Microadenomas are often difficult to detect unless rapid-sequence contrast-enhanced T1-weighted images are obtained. These tumors appear as foci of relatively low enhancement.
Macroadenomas
Macroadenomas show a heterogeneous enhancement pattern that reflects varying degrees of necrosis, cyst formation, and hemorrhage.
In macroadenomas, the aims of imaging are to precisely demarcate the boundary of normal tissue against the tumoral tissue, to assess for invasion of the cavernous sinus, and to demonstrate any mass effect on neighboring structures (eg, optic chiasm). Also critical is the relationship of the lesion to the nearby vasculature. These factors are important from the surgeon's perspective. Invasion of the cavernous sinus is related to biologically aggressive neoplasms and increases the risk of morbidity and mortality with surgical procedures even though the tumor remains histologically benign in most cases.
In a study by Connor et al, in 49 patients who had undergone transsphenoidal surgery for macroadenomas with potential unilateral parasellar involvement, the preoperative MR imaging features that were useful in predicting complete removal of the parasellar component of a pituitary adenoma (as assessed by postoperative MRI) were depiction of the lateral and inferolateral compartment of the cavernous sinus and decreasing encasement of the intracavernous carotid artery. [28]
According to Patel et al, postoperative early high-resolution volumetric MRI is valuable in determining the presence or absence of residual tumor after transsphenoidal pituitary surgery for macroadenomas. Early scans were 100% sensitive and 91% specific for predicting greater than 98% resection. The authors noted that cavity volume almost always decreases after surgery, and a lack of such a decrease should alert the surgeon to the possibility of persistent compression of the optic apparatus that may warrant reoperation. [29]
In one study, the use of 3T intraoperative MRI during transsphenoidal microsurgery on pituitary macroadenomas led to an increase in both the extent of tumor resection (in 8 of 73 patients, or 10.9%) and the rate of radical resections (69% instead of 60%). [30]
Enhanced imaging
High-resolution gadolinium-enhanced dynamic MRI has emerged as the most technically refined tool for evaluating pituitary microadenomas. This method makes use of the short temporal window in which contrast between the adenoma and the normal pituitary gland is high; this technique allows for optimal delineation of the tumor. This technique is superior to standard contrast-enhanced T1-weighted MRI in detecting microadenomas, particularly when no contour abnormality is present. This method also provides greater clarity than that of standard sequences after contrast agent is infused.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
On dynamic contrast-enhanced imaging, enhancement occurs in an expected sequence because of the unique and separate blood supplies to the pars nervosa, infundibulum, and pars distalis by the inferior hypophyseal artery, superior hypophyseal artery, and the portal system, respectively.
The earliest enhancement of normal structures is seen in the infundibulum and in the posterior lobe of the pituitary gland, followed by gradual enhancement of the anterior lobe from the junction of the infundibulum to the peripheral portion of the anterior lobe of the pituitary gland. Peak enhancement of pituitary adenomas usually occurs after the marked enhancement of the normal pituitary gland appears. By virtue of the differential enhancement pattern, adenomas are best seen in the early phase of gadolinium-enhanced dynamic imaging; they appear as hypointense lesions against the hyperintense background of the normally enhancing pituitary gland.
Other secondary morphologic features, such as infundibular displacement and focal glandular convexity, are readily demonstrable. Underlying bone destruction and tumoral calcification can be seen, but they are better demonstrated with CT than with MRI.
Degree of confidence
MRI is the examination of choice in pituitary and parasellar tumors because it depicts the complex anatomy around the sella well. Almost 30 various pathologic entities occur in this region, and most can be differentiated with MRI. These entities can be broadly divided on the basis of their involvement, as follows: intrasellar, infundibular, suprasellar, anterior part of the third ventricle and/or optic chiasm, and sphenoid and/or cavernous sinus. MRI is a good modality of choice to evaluate the organ of origin. [31, 32, 33, 34, 35, 36, 37, 38, 39]
Yin and associates studied the MRI data of 142 patients retrospectively with proven sellar lesions to evaluate the performance of MRI in the diagnosis of sellar lesions. Thirty patients were found to have pituitary adenoma cystic lesions, and sella were diagnosed in 24. Further classification resulted in 66 cases of parenchymatous lesions and 22 cases of cystic and parenchymatous lesions. The authors found MRI-based classification of sellar lesions useful for the diagnosis and differential diagnosis. [40]
According to Yun et al, based on a retrospective review of MRI scans and tumor histology of 50 patients who underwent endoscopic endonasal resection for nonfunctional adenomas, preoperative MRI analyses can be helpful, although not definitive, in predicting adenoma consistency. The investigators found that fibrous adenomas are associated with higher collagen content and are more difficult to resect, with higher rates of subtotal resection. [3]
False positives/negatives
In about 10% of the healthy adult population, MRIs show pituitary abnormalities compatible with the diagnosis of asymptomatic pituitary adenomas. Most pituitary adenomas remain asymptomatic and do not require treatment.
False-positive results are possible with asymptomatic focal pituitary lesions, such as cysts of the pars intermedia, metastases, infarctions, epidermoid cysts, and abscesses. These lesions may appear as areas of low signal intensity after the administration of gadolinium-based contrast agent. False-positive pituitary MRIs have also been reported in patients with ectopic ACTH secretion and the Cushing syndrome. In patients with Cushing syndrome, the incidence of pituitary adenomas at surgery is high; therefore, a focal hypointense area after gadolinium enhancement is accepted as being diagnostic of a pituitary microadenoma in a patient with pituitary endocrinopathy. [41]
Nuclear Imaging
Somatostatin receptors
Somatostatin receptors have been demonstrated in vitro in most GH-secreting pituitary adenomas and in some TSH- and PRL-secreting pituitary tumors. These receptors have not been consistently demonstrated in nonfunctioning adenomas. Recurrent or residual pituitary tumors are hard to distinguish from postoperative fibrosis with MRI and CT.
Scintigraphic visualization of residual tumor is possible with labeled somatostatin analogs, such as indium-111 diethylenetriaminepentaacetic acid (DTPA)-octreotide. This method allows scar tissue to be differentiated from recurrent tumor and helps in identifying patients who may benefit from somatostatin analogs.
Scintigraphy with technetium-99m pentavalent dimercaptosuccinic acid, or 99mTc (V)DMSA, also helps in detecting most pituitary GH- and PRL-secreting adenomas and nonfunctioning adenomas, with tumor-to-background ratios as high as 25. Functional imaging of pituitary remnant adenomas (>10 mm) with 99mTc (V)DMSA depicts viable remnants of pituitary adenomas. This approach may be of value in patients with clinically nonfunctioning adenomas to monitor the effects of radiation therapy. [42]
Indium-111-DTPA-octreotide scintigraphy is a technique for imaging somatostatin receptors in many neuroendocrine tumors (eg, pituitary adenomas). This agent is highly sensitive and a suitable tracer to evaluate the state of somatostatin receptor in pituitary adenomas. In one study, 111In-DTPA-octreotide single photon emission CT (SPECT) depicted adenomas in 21 (78%) of 27 patients who had 1 nonsecreting, 10 GH-secreting, and 10 PRL-secreting tumors.
The role of 111In-DTPA-octreotide scintigraphy in assessing nonfunctioning pituitary tumors has not been firmly established. Treatment with untagged octreotide may arguably interfere with tracer uptake in pituitary tumors. Therefore, patients undergoing scintigraphy should likely stop treatment for 2-3 days before the test. [43]
Studies have shown a good correlation between high 111In-DTPA-octreotide uptake and the response to octreotide therapy in patients with TSH- and GH-secreting pituitary adenomas. This method of imaging may facilitate the development of noninvasive treatments of somatostatin receptor–positive pituitary adenomas that might benefit from octreotide treatment. Such studies may be a breakthrough for poor surgical candidates, for patients who refuse surgery, or for presurgical and/or postsurgical imaging.
Naswa et al reported a case of multiple endocrine neoplasia type-1 syndrome associated with an ectopic pituitary adenoma in which somatostatin receptor–based PET/CT imaging using 68Ga-DOTANOC was instrumental in the diagnosis of ectopic pituitary adenoma. [44]
Angiography
Catheter angiography has a limited role in the management of pituitary adenomas. Conventional contrast-enhanced angiography is now virtually obsolete because MRA and CTA can depict the positions of the intracavernous and supraclinoid carotid arteries and because they are useful in differentiating pituitary mass lesions from aneurysms. In rare cases, the diagnoses of a substantially thrombosed aneurysm requires intra-arterial digital subtraction angiography (DSA).
Venography continues to have a role during catheter navigation for venous sampling during a workup for causes of Cushing syndrome. Bilateral simultaneous venous sampling from the inferior petrosal sinuses after a peripheral injection of corticotropin-releasing hormone (CRH) can confirm or rule out a central (pituitary) source of ACTH by showing a substantial gradient between the pituitary (central) and peripheral values of plasma ACTH and thus confirm Cushing disease.
Degree of confidence
The sensitivity and specificity of bilateral, simultaneous venous sampling from the inferior petrosal sinuses after a peripheral injection of CRH in confirming or ruling out a pituitary source of ACTH is 96-100%. The positive predictive value of this test is high because virtually no false-positive results occur. To some extent, it can even help in lateralizing the site of the pituitary tumor to guide microsurgery. False-positive results are virtually unknown with venous sampling from the inferior petrosal sinuses. False-negative findings are uncommon.
-
T1-weighted sagittal MRI through the pituitary fossa shows a normal, isointense anterior pituitary and a hyperintense posterior pituitary gland.
-
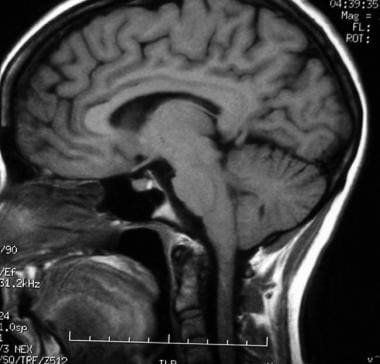
T1-weighted sagittal MRI through the pituitary fossa shows a normal pituitary gland.
-
T2-weighted axial MRI shows a normal optic chiasm and optic nerves.
-
Ectopic pituitary gland. Note the high signal intensity in median eminence and the absent normal posterior pituitary hyperintensity.
-
Sagittal T1-weighted MRI shows a nonenhancing pituitary macroadenoma.
-
Sagittal contrast-enhanced T1-weighted MRI shows a nonenhancing, hypointense lesion. Pituitary adenomas are most commonly hypointense on T1-weighted MRIs and hyperintense on T2-weighted MRIs.
-
Empty sella. Note the cerebrospinal fluid signal intensity in the herniated arachnoid space through the diaphragma sellae sella (which is partially filled with cerebrospinal fluid).
-
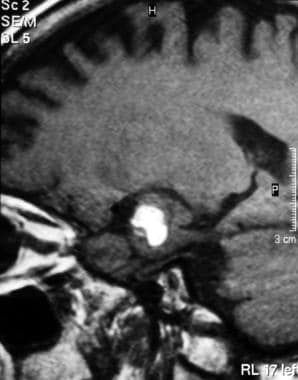
A 37-year-old woman presented with pituitary apoplexy. Note the high signal intensity in the pituitary gland secondary to hemorrhage on this T1-weighted MRI.
-
Sagittal T1-weighted MRI shows hemorrhage in a huge pituitary adenoma.
-
Sagittal contrast-enhanced T1-weighted MRI shows a huge, enhancing pituitary adenoma.
-
Coronal T1-weighted MRI shows a nonenhancing pituitary macroadenoma invading the left cavernous sinus (arrow).
-
Sagittal T2-weighted MRI shows a hyperintense cystic lesion above the pituitary gland due to a Rathke pouch cyst in the region of the pars intermedia.
-
Coronal T2-weighted MRI shows a large partially cystic craniopharyngioma that is causing obstructive ventriculomegaly.
-
Coronal fluid-attenuated inversion recovery (FLAIR) MRI of the brain shows an aneurysm of the intracranial carotid artery that is compressing the optic chiasm.
-
T1-weighted MRI shows a pearly, subarachnoid epidermoid cyst and high signal intensity.
-
Axial contrast-enhanced T1-weighted MRIs show an epidermoid cyst. Note the low signal intensity in the tumor along crevices of the subarachnoid space.
-
Sagittal T1-weighted image of the brain shows a hypothalamic hamartoma. Note the nonenhancing, small hypothalamic lesion with signal intensity similar to that of the gray matter in the region of the hypothalamus.
-
Axial contrast-enhanced T1-weighted MRIs show a metastasis to the sphenoid bone and the cavernous sinus from a primary site in the colon.
-
Coronal contrast-enhanced T1-weighted image shows meningeal nodular enhancement at the base of the brain due to sarcoidosis.
-
Axial contrast-enhanced T1-weighted MRI shows intense meningeal enhancement due to tuberculous meningitis. Note also the ring enhancement of a granuloma.
-
Coronal contrast-enhanced T1-weighted image shows intense enhancement in the region of the sphenoid and cavernous sinuses due to a fungal infection in a patient with immunocompromise.
-
Two coned lateral radiographs of the pituitary fossa show progression of disease. Left, Image shows ballooning of the pituitary fossa. Right, Image obtained 2 years latter shows bone destruction.
-
Lateral skull radiograph in a patient with pituitary adenoma shows an enlarged sella and focal calcification in the adenoma (arrow)
-
Radiograph shows a thickened heel pad in a patient with acromegaly.