Practice Essentials
Periventricular leukomalacia (PVL), or neonatal white matter injury, is the second most common central nervous system (CNS) complication in preterm infants, after periventricular hemorrhage. PVL is caused by ischemia in the watershed territory of the preterm infant. By definition, PVL has 2 neuropathologic components: a focal periventricular necrotic component and diffuse gliosis in the surrounding white matter. Cystic lesions secondary to necrotic foci in the white matter characterize the focal periventricular necrotic component. [1] The macroscopic focal necrotic component of PVL with diffuse gliosis is referred to as cystic PVL (c-PVL). In contrast, the term noncystic PVL is often used to denote the microscopic focal necrotic component of PVL plus a component of diffuse gliosis in cerebral white matter. [2, 3, 4, 5]
In the neonatal intensive care unit, serial cranial ultrasound (CUS) is the standard of care, because it is widely available, inexpensive, and noninvasive. The modality is good for assessment in the early stages of PVL, and the results are good predictors in subacute and chronic changes. Ultrasonography can make differentiation of the normal periventricular halo and homogeneous periventricular flares difficult, but MRIs do not show notable changes in the homogeneous flares. [6, 7, 8]
Ultrasound scans performed between weeks 2 and 6 help identify posthemorrhagic ventricular dilatation, white matter injury, focal arterial infarction, sequelae of brain infection, and rare cases of late intraventricular hemorrhage (IVH). Cystic white matter injury, or cystic periventricular leukomalacia, may become apparent within 14 days after injury, but small cysts may still occasionally develop for up to 6 weeks after birth. Serial scanning with a high-resolution probe (≥7.5 MHz) after 2 weeks of life is therefore necessary to identify all cases of periventricular leukomalacia. Scanning at the term-equivalent age allows the assessment of brain development and the identification of permanent residual injury of white matter iand gray matter. [7]
Because periventricular leukomalacia is a disease of premature infants, who often need the assistance of ventilators, ultrasonography is the easiest imaging modality to employ for examination. Ultrasonographic examination also provides an alternative to transporting the premature infant for examination with computed tomography (CT) scanning or MRI. MRI was originally thought to be superior to other investigations in only the subacute and chronic phases. [9, 10, 11] However, studies have shown that MRI is also good in identifying and characterizing acute changes. MRI shows extensive, large lesions and hemorrhages. The findings are also good predictors of the eventual outcome, in that patients with many changes in the acute stage eventually develop cystic changes. [12, 13, 14, 15, 16, 17]
A single examination may not be enough to diagnose PVL in early infancy. Diagnosis of PVL on ultrasound is only possible once cystic lesions develop, as it does not reliably detect diffuse white matter gliosis. [1] A combination of ultrasonography, MRI, and CT is useful for diagnosing PVL and for predicting the neurologic outcome. Ultrasonography is usually performed twice a week in the first week and once a week thereafter for diagnosis. Results of MRI performed between 12 and 18 months confirm the diagnosis, and the results are predictive of the ultimate neurologic outcome.
The magnetic resonance imaging (MRI) characteristics of periventricular leukomalacia are demonstrated in the images below. [18, 19]
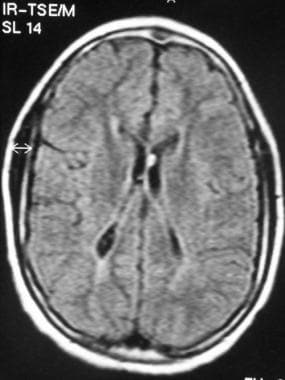 Acute stage of periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) MRI shows bilateral periventricular hyperintensity.
Acute stage of periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) MRI shows bilateral periventricular hyperintensity.
 High signal on a T1-weighted image obtained adjacent to the frontal horn of the right lateral ventricle. This is hemorrhagic periventricular leukomalacia (PVL).
High signal on a T1-weighted image obtained adjacent to the frontal horn of the right lateral ventricle. This is hemorrhagic periventricular leukomalacia (PVL).
Classification
PVL is classified as follows [1, 20] :
-
Grade I: Periventricular echodensities (7 days or more)
-
Grade II: Periventricular echodensities evolving into localized frontoparietal cystic lesions
-
Grade III: Extensive periventricular cystic lesions
-
Grade IV: Cystic lesions extend into deep white matter, subcortical cystic lesions
Computed Tomography
CT scanning shows ill-defined hypoattenuating ischemic areas in the periventricular white matter. This finding is difficult to distinguish in the highly hydrated, low attenuation of the unmyelinated premature brain matter. Hence, CT is not used in the diagnosis of periventricular leukomalacia. CT is most useful if hemorrhage is present. In severe injuries, extensive bilateral edema produces low attenuation, which produces ventricular compression. These findings are difficult to identify.
In the subacute stage, the ventricles have an irregular contour. The white matter is decreased in the periventricular region. Deep sulci are seen adjacent to the lateral ventricle due to a loss of white matter.
In the chronic stage, periventricular cysts are seen.
In the end stage, volume is lost along the lateral margins. Other findings are irregularly dilated ventricles and cortical gray matter extending deep toward ventricular walls. On gross inspection, an undulated ventricular wall is a typical feature. An elongated head due to loss of periventricular white matter is another feature. (A differential diagnosis is scaphocephaly.)
CT findings of end-stage PVL are irregular enlargement of the body and trigone of lateral ventricles; decreased white matter, which is conspicuous in the trigone but also in the centrum semiovale in severe cases; and deep sulci abutting the white matter due to loss of white matter.
The neurologic outcome can be predicted on the basis of the size and location of the cysts. Cysts smaller than 1 cm have a normal neurologic outcome. The risk of cerebral palsy is high in cysts larger than 1 cm, especially in those larger than 2 cm. Cysts located in only the frontal region do not affect the outcome. Cysts located bilaterally and in the parieto-occipital region pose a high risk of cerebral palsy.
The differential diagnosis of irregular ventricular walls includes PVL ventriculitis, ventricular hemorrhage, and disseminated tumor.
Magnetic Resonance Imaging
Detecting periventricular leukomalacia in acute stages is difficult because of the similar intensities of infarct and unmyelinated brain matter. However, T1-weighted MRIs sometimes show low signal intensity in the early stages, and T2-weighted MRIs show high signal intensity in the periventricular region. These appearances are due to periventricular ischemic changes. Diffusion images are more sensitive than these and show abnormal high signal due to restricted motion of intracellular water. These areas are seen as low signal on images of the apparent diffusion coefficient (ADC). MR spectroscopy can show high levels of lactate. [12, 14, 15, 16, 17]
Areas of hemorrhage are seen as high signal intensity on T1-weighted images (as seen in the image below) and low signal intensity on T2-weighted images. Punctate areas of high signal on T1-weighted MRIs that are in large areas of high signal intensity on T2-weighted MRIs are seen as early as 2-3 days after the injury. Low signal intensity on T2-weighted MRIs appears in 6-7 days.
 High signal on a T1-weighted image obtained adjacent to the frontal horn of the right lateral ventricle. This is hemorrhagic periventricular leukomalacia (PVL).
High signal on a T1-weighted image obtained adjacent to the frontal horn of the right lateral ventricle. This is hemorrhagic periventricular leukomalacia (PVL).
In the subacute phase, a high T1 signal can be due to transient calcification at the site of ischemia. Contrast enhancement is also seen.
Cysts subsequently form because of necrosis of periventricular tissue. The cysts shrink in 3-4 weeks, and the abnormal signal areas approach the ventricular wall and eventually disappear.
Sequelae of PVL (demonstrated in the images below) include atrophic changes in the cerebral parenchyma, loss of periventricular white matter, deep and prominent sulci that abut the ventricles with little or no white matter, irregularly dilated ventricles, cyst formation, and atrophy of the corpus callosum and thalamus. The volume of brainstem can be decreased. Thinning of corpus callosum is due to degeneration of transcallosal fibers and is most commonly seen in the splenium and posterior body.
 Late-stage periventricular leukomalacia (PVL). Asymmetrical loss of white matter, more on the right side than on the left, with a distorted frontal horn of the right lateral ventricle.
Late-stage periventricular leukomalacia (PVL). Asymmetrical loss of white matter, more on the right side than on the left, with a distorted frontal horn of the right lateral ventricle.
 Late-stage periventricular leukomalacia (PVL). Loss of periventricular white matter and dilated lateral ventricles.
Late-stage periventricular leukomalacia (PVL). Loss of periventricular white matter and dilated lateral ventricles.
 Severe late-stage periventricular leukomalacia (PVL). Extensive loss of white matter on the right side, with a grossly dilated frontal horn of the right lateral ventricle. Image shows atrophy of the brain parenchyma on both sides, although the atrophy is more marked on the right side than on the left. Note the sulci extending almost up to the ventricular margin; this is due to a loss of white-matter volume.
Severe late-stage periventricular leukomalacia (PVL). Extensive loss of white matter on the right side, with a grossly dilated frontal horn of the right lateral ventricle. Image shows atrophy of the brain parenchyma on both sides, although the atrophy is more marked on the right side than on the left. Note the sulci extending almost up to the ventricular margin; this is due to a loss of white-matter volume.
 Axial image shows a distorted right lateral ventricle and cortical atrophy with prominent and deep sulci.
Axial image shows a distorted right lateral ventricle and cortical atrophy with prominent and deep sulci.
 T2-weighted image in a patient with late-stage periventricular leukomalacia (PVL) shows a distorted occipital horn on the left side with atrophy and deep sulci that almost reach the ventricular margin.
T2-weighted image in a patient with late-stage periventricular leukomalacia (PVL) shows a distorted occipital horn on the left side with atrophy and deep sulci that almost reach the ventricular margin.
 Late-stage periventricular leukomalacia (PVL). Sagittal T1-weighted MRI shows an atrophied, irregular corpus callosum and atrophic brain parenchyma.
Late-stage periventricular leukomalacia (PVL). Sagittal T1-weighted MRI shows an atrophied, irregular corpus callosum and atrophic brain parenchyma.
 Sagittal T1-weighted MRI of late-stage periventricular leukomalacia (PVL) shows atrophy of the corpus callosum, cerebral parenchyma, and brainstem. Prominent sulci, many of which reach the ventricular margin, are depicted.
Sagittal T1-weighted MRI of late-stage periventricular leukomalacia (PVL) shows atrophy of the corpus callosum, cerebral parenchyma, and brainstem. Prominent sulci, many of which reach the ventricular margin, are depicted.
Other patterns of injury in ischemic hypoxic damage can also occur. These can involve necrosis in the basal ganglia, central cortical and subcortical damage, and multicystic encephalopathy.
Sie et al described an MRI grading system for PVL, as follows [21] :
-
Grade I - Normal MRI
-
Grade II - Altered periventricular intensity
-
Grade III - Fewer than 6 punctuate hemorrhages in the white matter
-
Grade IV - Multiple punctuate hemorrhages, a few large focal hemorrhages, and/or small periventricular cysts
-
Grade V - Extensive signal intensity changes within hemorrhagic and/or cystic lesions in the white matter, with minimal focal extension into the subcortical region
-
Grade VI - Diffuse signal intensity changes within hemorrhagic and/or cystic lesions in the white matter and subcortical region
Murgo et al described a combined ultrasonographic and MRI grading system, as follows [22] :
-
Grade I - Periventricular hyperechogenicity >1 week, with MRIs negative for PVL
-
Grade 2 - Periventricular hemorrhage and/or decreased white matter on MRIs
-
Grade 3 - Cysts
Argyropoulou et al demonstrated the following findings, which are due to the loss of white matter [23] :
-
Decrease in anteroposterior (AP) diameter of the pons
-
Decrease in the volume of the cerebellum
-
Decrease in the area of the corpus callosum
-
Decrease in the area of the vermis
The correlation between these measurements and the severity of PVL is good. The measurements are lowest in patients who received prolonged mechanical ventilation.
In end-stage PVL, T2-weighted MRI shows abnormally high signal intensity in the bilateral peritrigonal regions and delayed myelination, which is most common in patients with a young gestational age. This appearance resembles normally unmyelinated areas of white matter. However, a thin band of myelinated white matter in the splenium and tapetum separates these normal areas from the wall of ventricles.
The abnormal signal in PVL is in direct contact with the ventricular wall. These findings are best seen on coronal T2-weighted images and may not be seen on axial MRIs. The association with loss of volume of cerebral white matter and irregularly dilated ventricles are other helpful features. These changes are best seen on T2-weighted, proton density–weighted, or fluid-attenuated inversion recovery (FLAIR) images. If injury occurs in second trimester, loss of white matter occurs without gliosis. (A FLAIR image is shown below.)
 Acute stage of periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) MRI shows bilateral periventricular hyperintensity.
Acute stage of periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) MRI shows bilateral periventricular hyperintensity.
Degree of confidence
MRI is sensitive in the subacute and chronic phases of periventricular leukomalacia. The severity of changes on MRI is well correlated with neurologic outcomes, such as cerebral palsy, visual impairment, and delayed motor development. MRI is the best modality for assessing delayed myelination of the white matter.
However, Sie et al reported that MRI is useful even in the early acute stages of hypoxic-ischemic injury. [21] In their study, 64% of patients had hemorrhagic lesions, ranging from punctuate to gross lesions on MRI.
MRI is also useful for characterizing the exact site and extent of the PVL in the early stages, and it is better than ultrasonography for differentiating early stages. MRI shows more cysts than ultrasonography does. MRI shows changes in signal intensity, hemorrhages, and cysts, even when sonograms show only periventricular echogenicity. Findings on MRI are good predictors of the final ultrasonographic score. Moreover, MRI is superior to ultrasonography in predicting neurologic outcomes in patients with noncystic PVL, because ultrasonographic findings are nonspecific in these instances.
Interobserver and intraobserver correlation are better with MRI than with ultrasonography in cases of cystic PVL. The neurologic defect in noncystic PVL and the paucity of white matter are milder than those in cystic PVL.
The presence of hemorrhage on MRI is considered to be a bad prognostic factor. However, in some studies, hemorrhage was found frequently, and its exact prognostic significance is not yet clear.
The correlation between MRI changes in the acute stage and the eventual ultrasonographic grade is good. Patients with normal or minimal MRI changes do not develop severe cystic changes. However, patients with severe MRI changes eventually have severe cystic changes.
Many conditions produce periventricular T2 high signal and volume loss, including ventriculitis, inborn errors of metabolism, hydrocephalus, and in utero damage.
Ultrasonography
In preterm infants, ultrasonography is performed twice a week in the first week and once a week thereafter to detect periventricular hemorrhage and leukomalacia. In the neonatal intensive care unit, serial cranial ultrasound (CUS) is the standard of care, because it is widely available, inexpensive, and noninvasive. The modality is good for assessment in the early stages of PVL, and the results are good predictors in subacute and chronic changes. Ultrasonography can make differentiation of the normal periventricular halo and homogeneous periventricular flares difficult, but MRIs do not show notable changes in the homogeneous flares. [6, 7, 8]
Ultrasound scans performed between weeks 2 and 6 help identify posthemorrhagic ventricular dilatation, white matter injury, focal arterial infarction, sequelae of brain infection, and rare cases of late intraventricular hemorrhage (IVH). Cystic white matter injury, or cystic periventricular leukomalacia, may become apparent within 14 days after injury, but small cysts may still occasionally develop for up to 6 weeks after birth. Serial scanning with a high-resolution probe (≥7.5 MHz) after 2 weeks of life is therefore necessary to identify all cases of periventricular leukomalacia. Scanning at the term-equivalent age allows the assessment of brain development and the identification of permanent residual injury of white matter iand gray matter. [9, 10, 11, 16, 6, 7, 8]
Findings in the acute, subacute, and chronic stages
In the acute stage of PVL, sonograms show ill-defined, hyperechoic areas in the periventricular region. In comparison to the normal periventricular halo, this hyperechogenicity is brighter than that of choroid plexus. This appearance manifests 24-48 hours after the ischemic injury. It is particularly bright if periventricular hemorrhage is present. In the subsequent 1-2 weeks, these areas become less hyperechoic than before.
Although DiPietro et al indicated that irregular, inhomogeneous flares indicate hemorrhagic periventricular leukomalacia, hemorrhagic or nonhemorrhagic PVL are difficult to predict on the basis of its ultrasonographic appearance. [24] Sie et al found a direct correlation between irregular, inhomogeneous periventricular flares and the development of hemorrhagic and cystic changes on MRI. [21] Hence, the presence of an irregular flare is an important prognostic indicator.
In the subacute stage, the ventricular margins are irregular because of atrophic changes. In the chronic stage, well-defined, hypoechoic cysts are seen in the periventricular regions. The cysts are large if hemorrhage has occurred. The ventricles are large and irregular because of atrophic and ischemic changes. The cysts eventually disappear as the ventricles enlarge and the damaged tissue is reabsorbed.
Grading of periventricular densities and PVL
Periventricular densities and PVL are graded as follows [20] :
-
Grade I - Includes periventricular flares less than 1 week old
-
Grade II - Involves periventricular flares more than 1 week old (PVL grade I)
-
Grade III - Indicates small periventricular cysts (PVL grade II)
-
Grade IV - Defined as extensive periventricular cysts (PVL grade III)
-
Grade V - Multicystic leukomalacia in the periventricular and subcortical region (PVL grade IV)
In grade II, the cysts often develop after the first month, but in grade III, the cysts develop in the first 2-3 weeks. About 54% of all grade II cysts are unilateral despite the early appearance of bilateral periventricular densities. However, all grade III cysts are bilateral. Grade II cysts are most common in the anterior frontal periventricular region and least common in the parietal and occipital regions. More than 85% of grade III cysts are seen in the parieto-occipital periventricular region.
Cerebral palsy is less common in grade II PVL than in disease of other grades. The location of the cysts is not correlated with neurologic outcome in grade II, unlike grade III.
A modified ultrasonographic grading system is as follows [21] :
-
Grade IA - Includes transient flares involving periventricular densities less than 1 week old
-
Grade IB - Includes homogeneous periventricular densities more than 1 week old
-
Grade II - Includes inhomogeneous periventricular densities more than 1 week old
-
Grade III - Includes periventricular densities evolving into small cysts
-
Grade IV - Defined as periventricular densities evolving into extensive cysts
-
Grade V - Involves densities in the periventricular and subcortical regions with extensive and evolving periventricular and subcortical cysts
Ventriculomegaly indicates white-matter disease and is predictive of a poor neurologic outcome. Ventriculomegaly can also be a sequela of periventricular hemorrhage, which has a good prognosis. Ventricular enlargement is irregular in PVL and smooth in periventricular hemorrhage.
Degree of confidence
Ultrasonography is sensitive in the acute stage of PVL. Difficulties arise in small or bilaterally symmetrical cases. Affected areas also appear similar to normal areas of high echogenicity in the periventricular region. Ultrasonography is not as sensitive as MRI in the subacute and chronic stages. Ultrasonography can depict 28-80% of all cases of pathologically detected PVL. It is superior to MRI in the detection of cysts, although the cysts can be transient and missed on sonograms.
Ultrasonographic findings are well correlated with the neurologic outcome. The presence of cysts on sonograms indicates that the disease is severe and that the prognosis is poor, with adverse neurologic outcomes. The development of cysts, as shown on sonograms, is also correlated with volume loss on MRIs and a poor neurologic outcome.
Levine et al indicated that cranial ultrasonography performed at 40 weeks provides the best predictor of neurologic outcome. Almost all patients with bilateral frontal, occipital, and parietal white-matter lesions develop cerebral palsy; cerebral palsy develops in 35% of patients with small lesions and in 65% of patients with medium lesions. Irregular, inhomogeneous, periventricular echogenicities shown on sonograms and hemorrhage depicted on MRIs are well correlated with subsequent severe cystic PVL.
One of the difficulties is differentiating normal periventricular halos from periventricular flares of PVL. The periventricular flare of PVL is brighter than the choroid plexus, unlike the halo, which is not as bright.
The findings are operator dependent, and considerable intraobserver and interobserver variation can occur.
Ultrasonography is not good for assessing the exact grade of the PVL. It cannot be used to assess the cortical region and the structures of the posterior fossa.
Studies have shown that ultrasonography is inferior to MRI in assessing the exact extent and severity of PVL. It may be useful in premature infants younger than 29 weeks, in whom sonograms show only moderate homogeneous periventricular flares. However, Carson et al indicated that these changes are normal findings in very premature infants; therefore, MRIs are normal. [25]
-
Acute stage of periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) MRI shows bilateral periventricular hyperintensity.
-
High signal on a T1-weighted image obtained adjacent to the frontal horn of the right lateral ventricle. This is hemorrhagic periventricular leukomalacia (PVL).
-
Cystic change in periventricular white matter adjacent to the right lateral ventricle.
-
Mildly high signal intensity in the trigonal region secondary to periventricular leukomalacia (PVL).
-
Bilateral symmetrical periventricular hyperintensities and an enlarged occipital horn, especially on the right side.
-
Late-stage periventricular leukomalacia (PVL). Fluid-attenuated inversion recovery (FLAIR) image shows bilateral symmetrical periventricular hyperintensities.
-
Distorted occipital horns due to periventricular leukomalacia (PVL).
-
Late-stage periventricular leukomalacia (PVL). Asymmetrical loss of white matter, more on the right side than on the left, with a distorted frontal horn of the right lateral ventricle.
-
Late-stage periventricular leukomalacia (PVL). Loss of periventricular white matter and dilated lateral ventricles.
-
Severe late-stage periventricular leukomalacia (PVL). Extensive loss of white matter on the right side, with a grossly dilated frontal horn of the right lateral ventricle. Image shows atrophy of the brain parenchyma on both sides, although the atrophy is more marked on the right side than on the left. Note the sulci extending almost up to the ventricular margin; this is due to a loss of white-matter volume.
-
Axial image of late-stage periventricular leukomalacia (PVL) shows a dilated right occipital horn with irregular margins and periventricular high signal intensity.
-
Axial image shows a distorted right lateral ventricle and cortical atrophy with prominent and deep sulci.
-
T2-weighted image in a patient with late-stage periventricular leukomalacia (PVL) shows a distorted occipital horn on the left side with atrophy and deep sulci that almost reach the ventricular margin.
-
Late-stage periventricular leukomalacia (PVL). Sagittal T1-weighted MRI shows an atrophied, irregular corpus callosum and atrophic brain parenchyma.
-
Sagittal T1-weighted MRI of late-stage periventricular leukomalacia (PVL) shows atrophy of the corpus callosum, cerebral parenchyma, and brainstem. Prominent sulci, many of which reach the ventricular margin, are depicted.


