Practice Essentials
Bone metastases are common in patients with advanced solid tumors. Cancers that are particularly associated with bone metastases include those of the prostate and breast (65-75% of patients) and those affecting the lung (30-40%) and kidney (20-32%). [1] Early detection of skeletal metastasis is critical for accurate staging and optimal treatment. A substantial proportion of patients with advanced cancer and bone metastases develop skeletal-related events (SREs), including pathologic fracture, spinal cord compression, myelosuppression, and hypercalcemia. SREs are associated with moderate/severe pain, opioid analgesic use, reduced emotional well-being, and decreased survival. [2] Imaging has an important role in the detection, diagnosis, prognostication, treatment planning, and follow-up monitoring of bone metastases. In patients with proven nonskeletal tumors, imaging is useful for screening the skeleton to assess metastatic disease and, if it is present, to determine its extent. [3, 4, 5]
In a patient without a known malignancy, a possible diagnosis of bone metastases may be made by recognizing radiographic and other imaging findings. If bone metastases are present or suspected, further imaging or imaging-guided techniques may be required to confirm the diagnosis, to establish the extent of the disease, and to find the primary tumor.
Bone metastases are often multiple at the time of diagnosis. In adults, the lesions generally occur in the axial skeleton and other sites with residual red marrow, although the lesions may be found anywhere in the skeletal system. Common sites for metastases are the vertebrae, pelvis, proximal parts of the femur, ribs, proximal part of the humerus, and skull. More than 90% of metastases are found in this distribution.
Within the spine, most metastases are located in the lumbar spine, less frequently in the thoracic spine, and rarely in the cervical spine (52%, 36%, and 12%, respectively). [6] Certain tumors may have a predilection for particular skeletal sites. For example, metastases to the bones of the hands and feet are rare, but 50% of hand metastases originate from lung neoplasms (see the image below). Primary tumors arising from the pelvis have a predilection for spread to the lumbosacral spine.
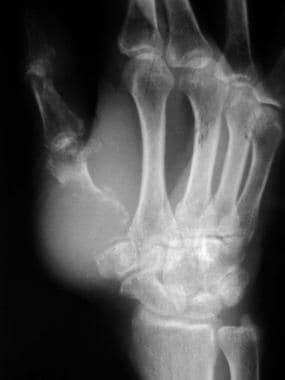 Bone metastases to the finger. Radiograph shows a destructive expanded osteolytic lesion in the metacarpal of the thumb in a 55-year-old man with lung carcinoma.
Bone metastases to the finger. Radiograph shows a destructive expanded osteolytic lesion in the metacarpal of the thumb in a 55-year-old man with lung carcinoma.
Occasionally, patients with bone metastases may present with a pathologic fracture; therefore, checking the state of underlying bone for disease is important if such a fracture is suspected (see the first image below). [7] In addition, patients may present with complications of bone metastases, such as neurologic impairment due to spinal epidural compression (see the second image below).
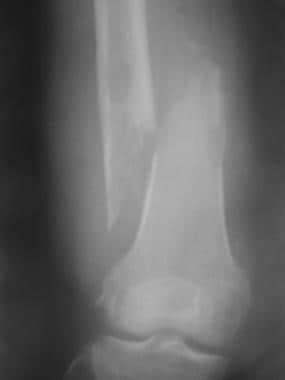 Pathologic fracture. Radiograph shows a displaced fracture through an osteolytic lesion in the distal femur of a 53-year-old woman with lung carcinoma.
Pathologic fracture. Radiograph shows a displaced fracture through an osteolytic lesion in the distal femur of a 53-year-old woman with lung carcinoma.
 Spinal epidural compression in a 70-year-old man with leg weakness. Lateral lumbar myelogram shows a complete epidural block due to a destructive osteolytic lesion of the L3 vertebral body. Lumbar puncture was performed at the L2-3 level.
Spinal epidural compression in a 70-year-old man with leg weakness. Lateral lumbar myelogram shows a complete epidural block due to a destructive osteolytic lesion of the L3 vertebral body. Lumbar puncture was performed at the L2-3 level.
Imaging modalities
Technetium-99m (99mTc) bone scintiscanning (ie, radionuclide bone scanning) is widely regarded as the most cost-effective and available whole-body screening test for the assessment of bone metastases. Conventional radiography is the best modality for characterizing lesions that are depicted on bone scintiscans. Combined analysis and reporting of findings on radiographs and 99mTc bone scintiscans improve the diagnostic accuracy in detecting bone metastases and assessing the response to therapy. [8, 9, 10]
Radiographs are relatively insensitive in the detection of early or small metastatic lesions. Although CT scans are superior to radiographs, CT scanning is also relatively insensitive in showing small intramedullary lesions, and it has the disadvantage of limited skeletal coverage. Bone scintiscan findings are sensitive but nonspecific. Whole-body MRI and fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning (FDG-PET) are accurate techniques that are limited by their high cost. [11, 12, 13, 14, 15]
CT scanning and MRI are useful in evaluating suspicious bone scintiscan findings that appear equivocal on radiographs. [16, 17, 18, 19] MRI can also help detect metastatic lesions before changes in bone metabolism make the lesions detectable on bone scintiscans. [20, 21, 22] CT scanning is useful in guiding needle biopsy, particularly in vertebral lesions. MRI is helpful in determining the extent of local disease in planning surgery or radiation therapy. [23, 24, 25, 26, 27, 28]
The first screening test used for the detection of bone metastases depends on the relative availability of MRI and 99mTc bone scintiscanning. The selection will become less of an issue when more MRI units are established and when its cost decreases. Factors such as cost and relatively long imaging times, as well as considerations of patient throughput, are important. MRI is estimated to cost 2-3 times as much as 99mTc bone scintigraphy [29, 30] ; fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning can cost 8 times as much. [25, 31, 32, 33, 34, 35, 36, 37, 38]
Whole-body MRI is increasingly being used to identify bone metastases, with a key component being diffusion-weighted imaging (DWI), which measures the microscopic diffusion properties of water to probe tissue microstructure. Metastatic bone lesions are typically hyperintense on DWI. MRI provides good contrast resolution of bone and soft tissue and therefore has good sensitivity and specificity for detection for bone metastases. [39, 40, 41]
Fluorine-18–labeled sodium fluoride (18F-NaF) PET/CT has been shown to provide many advantages, such as early detection, accurate information about the extent of metastatic bone lesions, and excellent image quality (4-5 mm spatial resolution). However, it is not tumor-specific and, therefore, has a lower specificity for ruling out metastatic skeletal involvement. [42, 43, 44, 45, 46]
Conventional angiography is performed using fluoroscopy. Many use fluoroscopy to guide procedures, but it has little use in bone tumor imaging, except to localize bone tumors at the time of surgery and in chemoembolization treatments and preoperative embolization of vascular tumors. [47]
Ultrasound imaging is a noninvasive and inexpensive imaging modality to detect a lesion, pinpoint location, establish vascularity, and determine if it is cystic or solid. Since the ultrasound beam cannot penetrate the bone, it is more useful in soft-tissue lesions. However, it is effective in detecting extraosseous tumor extension and guiding biopsy or procedures. [47]
Pathophysiology
A basic knowledge of the processes by which metastases involve bone helps in understanding radiologic findings. Bone involvement in metastases occurs by means of 3 main mechanisms: (1) direct extension, (2) retrograde venous flow, and (3) seeding with tumor emboli via the blood circulation. Seeding occurs initially in the red marrow; this process accounts for the predominant distribution of metastatic lesions in the red marrow–containing areas in adults. In contrast, bone metastases are usually widespread in children. Retrograde venous embolism is probably the major mechanism when spread from intra-abdominal cancer involves the vertebrae. Increased intra-abdominal pressure causes blood to be diverted from the systemic caval system to the valveless vertebral venous plexus of Batson; this diversion allows the caudal and cranial flow of blood.
As a metastatic lesion grows in the medullary cavity, the surrounding bone is remodeled by means of either osteoclastic or osteoblastic processes. Metastases from kidney, thyroid, and lung malignancies are predominantly osteolytic, while osteoblastic lesions are usually seen in prostate cancer and breast cancer. The relative degree of resultant bone resorption or deposition is highly variable and depends on the type and location of the tumor. The relationship between the osteoclastic and osteoblastic remodeling processes determines whether a predominant lytic, sclerotic, or mixed pattern is seen on radiographs. [48]
Radiography
Radiography remains the best method for characterizing bone metastases. Bone metastases may be osteolytic, sclerotic, or mixed on radiographs (see the first image below). Lesions usually appear in the medullary cavity, spread to destroy the medullary bone, and then involve the cortex. Osteolytic metastases are encountered most frequently, especially in breast and lung carcinomas (see the second image below). [49, 50, 51, 52] The specific appearance of bone metastases is often useful in suggesting the nature of the underlying primary malignancy.
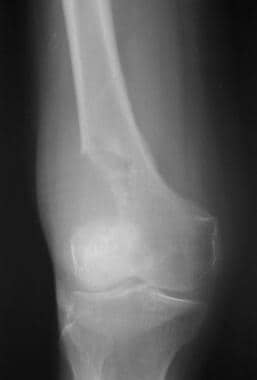 Radiograph shows osteolytic metastasis in the distal femur of a 51-year-old woman with breast carcinoma.
Radiograph shows osteolytic metastasis in the distal femur of a 51-year-old woman with breast carcinoma.
Metastases from certain primary sites (eg, renal cell or thyroid carcinomas) are almost always osteolytic, whereas those from other sites (eg, prostatic carcinoma) are predominantly sclerotic (see the image below). [53] Other malignancies associated with sclerotic metastases include breast carcinoma, colon carcinoma, melanoma, bladder carcinoma, and soft-tissue sarcoma. The findings of sclerotic metastases virtually exclude an untreated renal tumor or hepatocellular carcinoma. [54]
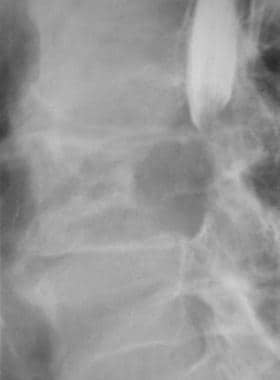
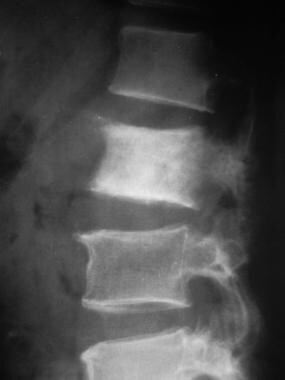 Lateral radiograph shows sclerotic metastasis of the L2 vertebra in a 54-year-old man with prostatic carcinoma.
Lateral radiograph shows sclerotic metastasis of the L2 vertebra in a 54-year-old man with prostatic carcinoma.
In vertebrae, clues to metastatic involvement include pedicular destruction, an associated soft-tissue mass, and an angular or irregular deformity of the vertebral endplates.
The response to therapy can be evaluated by using radiographs and by correlating the radiographic changes with bone scintiscan findings and clinical and laboratory data. The initial manifestation of healing in an osteolytic metastatic lesion is a sclerotic rim of reactive bone. With progressive healing, sclerosis increases and advances from periphery of the lesion to its center: The lesion shrinks and eventually resolves. For a mixed osteolytic-sclerotic lesion, a healing response to therapy is demonstrated as uniform lesional sclerosis, whereas increasing osteolysis indicates disease progression.
Purely sclerotic lesions are more difficult to assess. A sclerotic lesion that shrinks or completely disappears after therapy signifies disease regression, whereas one that grows and causes destruction implies progression. The comparison of current images with previous radiographs is essential, particularly in the detection of subtle lesional changes.
Degree of confidence
Compared with other imaging techniques, radiography is relatively insensitive in detecting bone metastases, especially subtle lesions. As a general rule, only lesions 2 cm or larger are radiographically apparent. Metastases to bone become apparent on radiographs only after the loss of more than 50% of the bone mineral content at the site of disease.
On radiographs, advanced destructive lesions of the cancellous bone may not be visible, particularly in the absence of reactive new bone or cortical involvement. This problem is more apparent in elderly patients with osteopenic bones than in others.
Osteolytic metastases can mimic osteoarthritis both clinically and radiographically; for example, they can mimic subchondral cysts and Schmorl nodes in the spine. Osteolytic foci may resemble amyloidosis, cystic angiomatosis, and infiltrative bone marrow lesions. Sclerotic metastases may be difficult to distinguish from other sclerotic bone lesions, such as bone islands, tuberous sclerosis, mastocytosis, and osteopoikilosis.
In assessing the response to therapy, an increasing number of sclerotic bone metastases may be difficult to distinguish from the healing of sclerotic lesions that were not previously identified.
Computed Tomography
CT scans are valuable in the evaluation of focal abnormalities seen on bone scintiscans that cannot be confirmed by using radiographs. Moreover, CT scanning is useful in further assessment of radiographically negative areas in patients who are symptomatic and in whom metastases are suggested clinically. Osteolytic, sclerotic, and mixed lesions are depicted well on CT scans (see the image below).
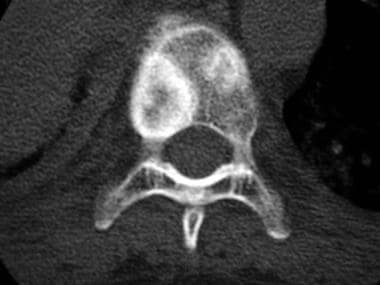 Axial computed tomography scan shows 2 rounded, mixed osteolytic-sclerotic lesions in the thoracic vertebral body of a 44-year-old woman with lung carcinoma.
Axial computed tomography scan shows 2 rounded, mixed osteolytic-sclerotic lesions in the thoracic vertebral body of a 44-year-old woman with lung carcinoma.
CT is useful in guiding needle biopsy of lesions in bones with complex shapes, such as the vertebrae and the ilia (see the image below).
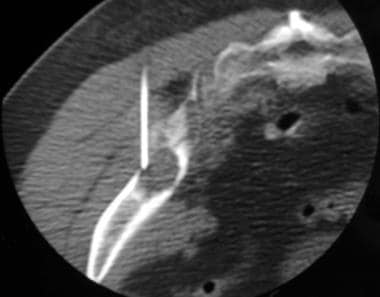 Computed tomography (CT)-guided biopsy was performed in the left ilium of a 50-year-old woman with an unknown primary tumor. This axial CT scan, obtained with the patient lying prone, shows the tip of the 17-gauge bone biopsy needle in the osteolytic lesion. Histologic analysis demonstrated adenocarcinoma of the lung.
Computed tomography (CT)-guided biopsy was performed in the left ilium of a 50-year-old woman with an unknown primary tumor. This axial CT scan, obtained with the patient lying prone, shows the tip of the 17-gauge bone biopsy needle in the osteolytic lesion. Histologic analysis demonstrated adenocarcinoma of the lung.
Skeletal coverage is limited with CT scanning because of its relatively high radiation dose, which makes this imaging modality unsuitable as a screening tool.
The usefulness of CT scanning in detecting early deposits in bone marrow is limited. Muindi et al and Durning et al found that CT scanning is more sensitive than radiography in the detection of metastatic lesions. [55, 56] In fact, CT scanning is vastly superior to radiography in the detection of trabecular and cortical bone destruction, soft-tissue extension, and involvement of neurovascular structures (see the image below).
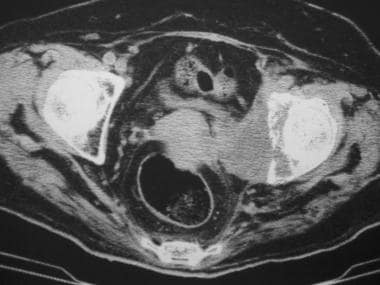 Axial computed tomography scan shows a destructive osteolytic lesion in the left acetabulum of a woman with vulval carcinoma. Soft-tissue extension into the pelvic cavity is present.
Axial computed tomography scan shows a destructive osteolytic lesion in the left acetabulum of a woman with vulval carcinoma. Soft-tissue extension into the pelvic cavity is present.
Although CT scanning is superior to radiography, some advanced destructive lesions of the cancellous bone may not be visible on CT scans, particularly in the absence of reactive new bone or cortical involvement.
Magnetic Resonance Imaging
Many authors have shown that MRI is more sensitive than technetium-99m (99mTc) bone scintiscanning in the detection of bone metastases. However, the use of MRI to screen the skeleton has long been regarded as impractical, although Steinborn et al and Eustace et al have shown that whole-body MRI is a feasible alternative to 99mTc planar bone scintiscanning in evaluating the entire skeleton for metastatic disease. [57, 58] Whole-body MRI requires 40-45 minutes to perform and involves the use of short-tau inversion recovery (STIR) and/or T1-weighted sequences. [59]
Metastatic seeding in the bone marrow is characterized by long T1 relaxation times, whereas T2 relaxation times are variable, depending on tumor morphology. Lesions are seen as focal or diffuse areas of hypointensity on T1-weighted images and as areas of intermediate or high signal intensity on T2-weighted images. Tumor deposits typically appear hyperintense against a dark background of suppressed signal intensity within fat on STIR images (see the images below).
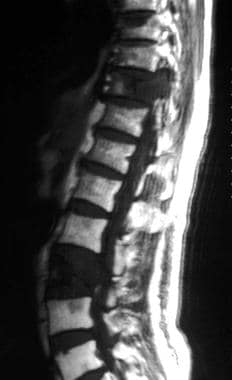 Sagittal spin-echo T2-weighted magnetic resonance image shows hypointense lesions in the T10 and L3 vertebrae in a 66-year-old man with lung carcinoma. The tumor involves the T10 pedicle. See also the next image.
Sagittal spin-echo T2-weighted magnetic resonance image shows hypointense lesions in the T10 and L3 vertebrae in a 66-year-old man with lung carcinoma. The tumor involves the T10 pedicle. See also the next image.
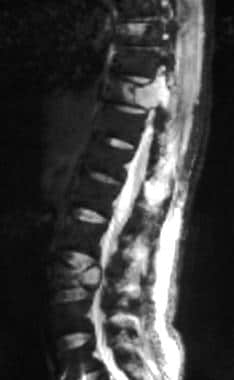 Sagittal short-tau inversion recovery magnetic resonance (MRI) from a 66-year-old man with lung carcinoma. This MRI shows hyperintense lesions in the T10 and L3 vertebrae, with T10 pedicular involvement.
Sagittal short-tau inversion recovery magnetic resonance (MRI) from a 66-year-old man with lung carcinoma. This MRI shows hyperintense lesions in the T10 and L3 vertebrae, with T10 pedicular involvement.
The bull's-eye or halo sign has been reported to be useful in distinguishing metastatic from benign lesions. [60] In vertebrae, additional criteria for malignancy include bulging of the posterior margin of the vertebral body, signal intensity changes that extend into the pedicle, and paraosseous tumor spread (see the images below).
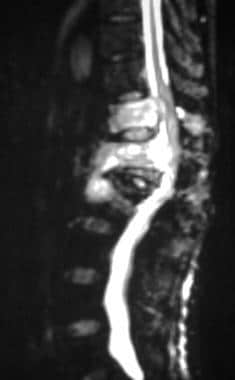 Sagittal short-tau inversion recovery (STIR) magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows severe compression of the L1 vertebra with retropulsion. Affected T11-L2 vertebrae show signal hyperintensity, posterior vertebral body marginal bulging, and spinal canal narrowing. See also the next image.
Sagittal short-tau inversion recovery (STIR) magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows severe compression of the L1 vertebra with retropulsion. Affected T11-L2 vertebrae show signal hyperintensity, posterior vertebral body marginal bulging, and spinal canal narrowing. See also the next image.
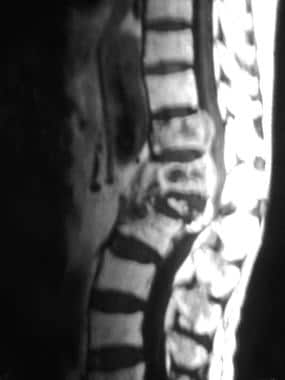 Sagittal gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the T11-L2 vertebrae, with prominent epidural component enhancement and spinal canal compromise. See also the next image.
Sagittal gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the T11-L2 vertebrae, with prominent epidural component enhancement and spinal canal compromise. See also the next image.
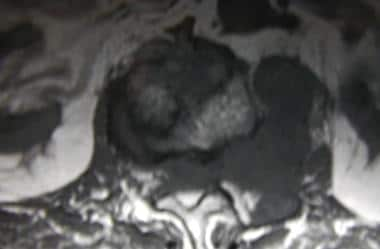 Axial spin-echo T1-weighted magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows tumor extension from the L1 vertebral body and left pedicle into the left psoas muscle and epidural space, with resultant spinal cord compression. See also the next image.
Axial spin-echo T1-weighted magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows tumor extension from the L1 vertebral body and left pedicle into the left psoas muscle and epidural space, with resultant spinal cord compression. See also the next image.
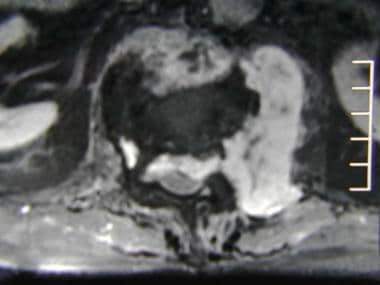 Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the soft tissue component of the L1 vertebral metastatic tumor.
Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the soft tissue component of the L1 vertebral metastatic tumor.
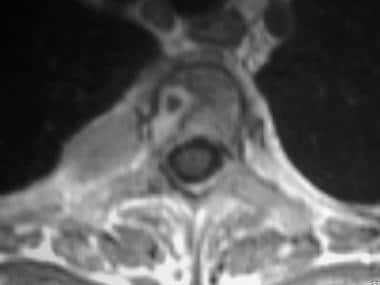 Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image in a 43-year-old woman with breast carcinoma. This image of the T3 vertebra shows a ring-enhancing lesion and an expansile metastatic left rib deposit.
Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image in a 43-year-old woman with breast carcinoma. This image of the T3 vertebra shows a ring-enhancing lesion and an expansile metastatic left rib deposit.
Gadolinium-based contrast agents have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or MRA scans. NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
Degree of confidence
MRI depicts early hematogenous dissemination of the tumor to the bone marrow before reactions in adjacent bone are detectable on 99mTc scintiscans. Many studies have shown that MRI is more sensitive than 99mTc bone scintiscanning in the detection of bone metastases. Steinborn et al reported sensitivities of 91.4% for MRI and 84.8% for bone scintiscanning. [57] Flickinger and Sanal reported sensitivities of 100% for MRI and 62% for scintiscanning and specificities of 62% for MRI and 100% for scintiscanning. [61] For MRI and scintiscans, respectively, Eustace et al reported sensitivities of 96.5% and 72%, specificities of 100% and 98%, and positive predictive values of 100% and 95%. [58]
False positives/negatives
Distinguishing between benign and malignant causes of vertebral compression fractures may be difficult. [62] Baur et al and other investigators have reported on the use of MRI diffusion-weighted imaging (DWI) in discriminating between benign and malignant acute vertebral body compression fractures. [48, 63, 64, 65, 66] Their findings were supported by the work of Spuentrup et al, [67] whereas Castillo et al found that DWI offered no additional advantage. [68]
DWI is highly sensitive to cellularity and the mobility of free water molecules, which are responsible for the differences between benign and malignant fractures. Using a single-shot echo-planar DWI pulse sequence with a high b factor, Chan et al showed that apparent diffusion coefficient values are useful in differentiating benign osteoporotic fractures from malignant vertebral body compression fractures. [69]
Nuclear Imaging
Technetium-99m (99mTc) bone scintigraphy is an effective method for screening the whole body for bone metastases. [70, 71] Tc-99m diphosphonates, most commonly 99mTc methylene diphosphonate (MDP), is the most frequently used isotope. Tc-99m planar bone scintiscans help detect metastatic bone deposits by the increased osteoblastic activity they induce; this finding is considered an indirect marker of tumor.
Indications for bone scintiscanning include staging in asymptomatic patients; evaluating persistent pain in the presence of equivocal or negative radiographic findings; determining the extent of bone metastases in patients with positive radiographic findings; differentiating metastatic from traumatic fractures by assessing the pattern of involvement; and determining the therapeutic response to metastases.
PET scanning can help identify bone metastases at an early stage of growth before host reactions to the osteoblasts occur. FDG-PET scanning depicts early malignant bone-marrow infiltration because of the early increased glucose metabolism in neoplastic cells. [72]
Isotopic imaging methods depict bone metastatic lesions as areas of increased tracer uptake. The classic pattern appears as the presence of multiple randomly distributed focal lesions throughout the skeleton (see the first image below). Findings of a solitary scintigraphic abnormality or just a few lesions may present special problems in the interpretation of findings. Other patterns include diffuse involvement (superscan), photopenic lesions (cold lesions), normal scintiscans, flare phenomena, and soft-tissue lesions (see the second image below). [70]
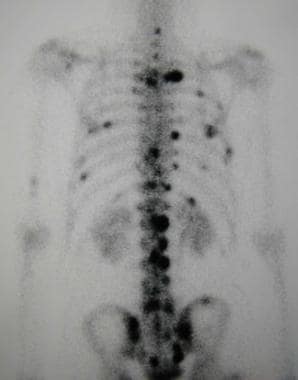 Typical scintigraphic pattern of bone metastases in a 60-year-old man with nasopharyngeal carcinoma. This posterior technetium-99m bone scintiscan shows multiple randomly distributed focal lesions scattered throughout the skeleton, particularly the spine, ribs, and pelvis.
Typical scintigraphic pattern of bone metastases in a 60-year-old man with nasopharyngeal carcinoma. This posterior technetium-99m bone scintiscan shows multiple randomly distributed focal lesions scattered throughout the skeleton, particularly the spine, ribs, and pelvis.
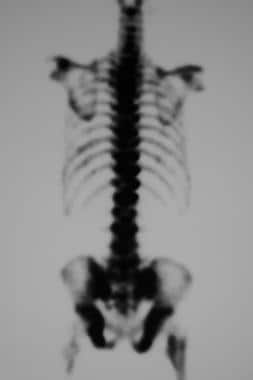 Posterior technetium-99m bone scintiscan in a 79-year-old man with prostatic carcinoma. This image shows diffuse and intense uptake in most of the bones. Patchy rib lesions are also seen, but renal uptake is absent.
Posterior technetium-99m bone scintiscan in a 79-year-old man with prostatic carcinoma. This image shows diffuse and intense uptake in most of the bones. Patchy rib lesions are also seen, but renal uptake is absent.
There is increased interest in additional tumor-specific tracers that relate to tumor metabolism and antigen expression, such as choline labeled with either 11C-carbon or 18F-fluorine. Uptake is seen in osteoblastic metastases, with even higher activity noted in rarer osteolytic phenotypes. 68Ga-prostate-specific membrane (68Ga-PSMA) antigen tracers have shown advantages in sensitivity, specificity, and tumor-to-background contrast (particularly in the skeleton) in comparison to 18F-choline PET/CT. 68Ga-PSMA has been shown to be superior to bone scintigraphy in primary staging. [73, 74, 75, 76, 77]
Degree of confidence
Tc-99m bone scintiscan findings are nonspecific in determining the cause of increased uptake, particularly in solitary lesions. Bone scintiscans have the disadvantages of poor spatial and contrast resolution. In many patients, further imaging is required to characterize regions of disseminated abnormality. Despite the superior sensitivity of MRI compared to bone scintiscanning, bone scintiscanning continues to be used as the initial screening investigation because of its relatively low cost, wide availability, and usefulness in imaging the entire skeleton.
FDG-PET scanning has limited spatial resolution, and complementary CT scanning or MRI is required to localize an area of increased glucose metabolism.
False positives/negatives
Sensitivities of 99mTc bone scintiscans are reportedly 62-89%. Many benign processes and normal variants can produce an area of increased isotope uptake that mimics a metastatic deposit. Solitary areas of abnormal uptake associated with benign processes occur in approximately one third of patients with malignant disease.
The differential diagnosis of multiple scintigraphic abnormalities includes metabolic problems (eg, Cushing syndrome), osteomalacia, trauma, arthritis, osteomyelitis, Paget disease, and infarctions. Some metastases may produce normal scintiscan findings. Cold or photopenic metastases may be found in association with lesions of highly aggressive anaplastic carcinomas.
In diffuse metastatic disease, isotopic accumulation may be sufficiently uniform to produce a false-negative impression. Clues to the detection of the so-called superscan include skeletal uptake of greater-than-normal intensity, in relation to the background of the soft tissue and the low or absent uptake in the kidneys (see the image below).
 Posterior technetium-99m bone scintiscan in a 79-year-old man with prostatic carcinoma. This image shows diffuse and intense uptake in most of the bones. Patchy rib lesions are also seen, but renal uptake is absent.
Posterior technetium-99m bone scintiscan in a 79-year-old man with prostatic carcinoma. This image shows diffuse and intense uptake in most of the bones. Patchy rib lesions are also seen, but renal uptake is absent.
Osteoblastic activity that reflects attempts at bone healing after chemotherapy (ie, flare phenomenon) may misleadingly suggest advancing disease on scintigraphy. The number of false-positive scintiscans can be decreased if the findings are reviewed with the corresponding radiographs.
In a comparative study of 3 modalities, Daldrup-Link et al found sensitivities of 90% for FDG-PET scanning, 82% for whole-body MRI, and 71% for 99mTc bone scintiscanning. [78] Similar results have emerged from comparative studies of FDG-PET scanning and 99mTc bone scintiscans. [79, 80] However, Franzius et al found that FDG-PET scanning is less sensitive than bone scintiscanning in the differentiation of bone metastases from osteosarcoma. [81]
FDG-PET scanning shows a high number of false-positive lesions, which require follow-up imaging with other modalities. The use of semiquantitative criteria for tumor FDG uptake in the qualitative evaluation of images may increase the specificity. In skull metastases, the high rate of glucose metabolism in the normal areas of brain may obscure metastases.
18F-Sodium Fluoride (18F-NaF) PET/CT
18F-Sodium fluoride (18F-NaF) PET/CT bone scanning allows high-resolution functional imaging of bone metastases with significantly greater sensitivity (100%) and specificity (97%) than conventional planar bone scintigraphy. [42] The pharmacokinetics of 18F-NaF, including nearly 100% first-pass extraction into bone, negligible protein binding, and rapid renal excretion in hydrated subjects, allow imaging of the skeleton with high contrast and spatial resolution in less than 1 hour after injection. [82] Another advantage of 18F-NaF PET/CT is that low-dose CT scan reduces the need for radiographs, diagnostic CT, or MRI scans to exclude metastatic disease in equivocal cases. [42]
A retrospective analysis of 31 individuals with medullary thyroid cancer (MTC) who underwent 18F-NaF PET/CT to evaluate for bone metastases reported that bone lesions were identified in 62% of patients in areas not assessed by standard MRI studies. [83]
The disadvantages of 18F-NaF PET/CT are more false-positive results because there is a tendency to pick up benign pathology; false-negative scans occasionally occur if there is a solitary small lytic metastasis in the bone marrow with little associated osteoblastic activity. There is an increased total effective radiation dose to the patients, and interpretation of the scans requires more time because their greater sensitivity picks up more findings and the CT portion must be viewed in detail. [42]
Another limitation is that 18F-NaF PET/CT does not detect metastases in lymph nodes or viscera. [84, 85]
A retrospective study by Panangiotidis et al of 66 patients who underwent spinal MRI and 18F-NaF PET/CT for restaging of recurrent high-risk breast cancer found that 18F-NaF PET/CT and spinal MRI were concordant in 51 patients (77.3%). In the other patients, 18F-NaF PET/CT detected more lesions in 4 patients (7.6%), and MRI detected more lesions in 10 patients (15.1%). The high level of concordance suggested that there is no need to perform both tests. [86]
-
Bone metastases to the finger. Radiograph shows a destructive expanded osteolytic lesion in the metacarpal of the thumb in a 55-year-old man with lung carcinoma.
-
Pathologic fracture. Radiograph shows a displaced fracture through an osteolytic lesion in the distal femur of a 53-year-old woman with lung carcinoma.
-
Spinal epidural compression in a 70-year-old man with leg weakness. Lateral lumbar myelogram shows a complete epidural block due to a destructive osteolytic lesion of the L3 vertebral body. Lumbar puncture was performed at the L2-3 level.
-
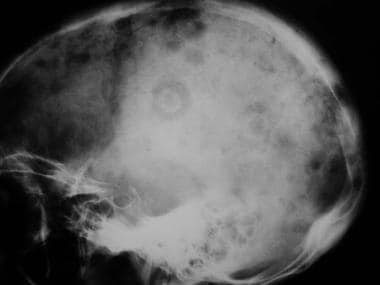
Lateral radiograph shows mixed osteolytic-sclerotic bone metastases in the skull vault.
-
Radiograph shows osteolytic metastasis in the distal femur of a 51-year-old woman with breast carcinoma.
-
Lateral radiograph shows sclerotic metastasis of the L2 vertebra in a 54-year-old man with prostatic carcinoma.
-
Axial computed tomography scan shows 2 rounded, mixed osteolytic-sclerotic lesions in the thoracic vertebral body of a 44-year-old woman with lung carcinoma.
-
Axial computed tomography scan shows a destructive osteolytic lesion in the left acetabulum of a woman with vulval carcinoma. Soft-tissue extension into the pelvic cavity is present.
-
Computed tomography (CT)-guided biopsy was performed in the left ilium of a 50-year-old woman with an unknown primary tumor. This axial CT scan, obtained with the patient lying prone, shows the tip of the 17-gauge bone biopsy needle in the osteolytic lesion. Histologic analysis demonstrated adenocarcinoma of the lung.
-
Sagittal spin-echo T2-weighted magnetic resonance image shows hypointense lesions in the T10 and L3 vertebrae in a 66-year-old man with lung carcinoma. The tumor involves the T10 pedicle. See also the next image.
-
Sagittal short-tau inversion recovery magnetic resonance (MRI) from a 66-year-old man with lung carcinoma. This MRI shows hyperintense lesions in the T10 and L3 vertebrae, with T10 pedicular involvement.
-
Sagittal short-tau inversion recovery (STIR) magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows severe compression of the L1 vertebra with retropulsion. Affected T11-L2 vertebrae show signal hyperintensity, posterior vertebral body marginal bulging, and spinal canal narrowing. See also the next image.
-
Sagittal gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the T11-L2 vertebrae, with prominent epidural component enhancement and spinal canal compromise. See also the next image.
-
Axial spin-echo T1-weighted magnetic resonance image (MRI) in a 68-year-old man with thyroid carcinoma. This MRI shows tumor extension from the L1 vertebral body and left pedicle into the left psoas muscle and epidural space, with resultant spinal cord compression. See also the next image.
-
Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image from a 68-year-old man with thyroid carcinoma. This image shows heterogeneous enhancement of the soft tissue component of the L1 vertebral metastatic tumor.
-
Axial gadolinium-enhanced spin-echo T1-weighted magnetic resonance image in a 43-year-old woman with breast carcinoma. This image of the T3 vertebra shows a ring-enhancing lesion and an expansile metastatic left rib deposit.
-
Typical scintigraphic pattern of bone metastases in a 60-year-old man with nasopharyngeal carcinoma. This posterior technetium-99m bone scintiscan shows multiple randomly distributed focal lesions scattered throughout the skeleton, particularly the spine, ribs, and pelvis.
-
Posterior technetium-99m bone scintiscan in a 79-year-old man with prostatic carcinoma. This image shows diffuse and intense uptake in most of the bones. Patchy rib lesions are also seen, but renal uptake is absent.