Practice Essentials
Yellow fever is a mosquito-borne disease that is endemic to tropical South America and sub-Saharan Africa (see the image below). Its presentation can range from asymptomatic illness to acute-onset viral hepatitis and hemorrhagic fever. [1, 2, 3]
 This female Aedes aegypti mosquito is shown after landing on a human host. The A aegypti mosquito is a known transmitter of dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when he or she has been bitten by a female mosquito. Image courtesy of the CDC/World Health Organization (WHO).
This female Aedes aegypti mosquito is shown after landing on a human host. The A aegypti mosquito is a known transmitter of dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when he or she has been bitten by a female mosquito. Image courtesy of the CDC/World Health Organization (WHO).
Signs and symptoms
History
Yellow fever usually is a mild, self-limited illness consisting of fever, headache, myalgia, and malaise. It typically is divided into three stages: the period of infection, period of remission, and period of intoxication. [3]
More serious illness presents with the abrupt onset of the following during the period of infection:
-
General malaise
-
Fever
-
Chills
-
Headache
-
Lower back pain
-
Nausea
-
Dizziness
This is followed by a period of remission, when the virus is cleared and when many infected individuals fully recover.
In 15-25% of individuals with yellow fever, symptoms recur during the period of intoxication and can progress to fatal illness. This period is marked by the following [3] :
-
Fever
-
Vomiting
-
Abdominal pain
-
Renal failure
-
Hemorrhage
Physical examination
Physical findings in yellow fever include the following [3] :
-
Fever
-
Relative bradycardia for the degree of fever (Faget sign)
-
Conjunctival injection
-
Skin flushing
As the disease progresses, additional physical findings include the following [3] :
-
Scleral icterus
-
Jaundice
-
Epigastric tenderness
-
Hepatomegaly
The following is also often apparent in severe disease [3] :
-
Petechiae
-
Purpura
-
Mucosal bleeding
-
Gastrointestinal bleeding (gross or occult)
Organ ischemia, which primarily affects the kidneys and central nervous system, leads to altered mental status and/or signs of volume overload. In the late stages of yellow fever, patients present with the following [3] :
-
Tachycardia
-
Hypothermia or hyperthermia
-
Hypotension
Individuals who are severely hypoperfused appear mottled and cyanotic; they are also often obtunded. Tachypnea and hypoxia with impending respiratory failure may develop as a consequence of sepsis and acute respiratory distress syndrome (ARDS).
See Clinical Presentation for more detail.
Diagnosis
Laboratory studies
-
Complete blood count (CBC)
-
Chemistries
-
Liver function tests (LFTs): Elevated levels of transaminases precede the appearance of jaundice, and the degree of liver dysfunction in the acute phase may be predictive of the clinical course.
-
Coagulation studies
-
Urinalysis
Imaging studies
Chest radiography is used to evaluate the extent of pulmonary edema, to reveal secondary bacterial pulmonary infections, and to aid in ventilator management if intubation is required.
Specific tests for yellow fever
-
Rapid detection methods (eg, polymerase chain reaction assay)
-
Serologic tests (eg, enzyme-linked immunosorbent assay)
-
Immunohistochemical tissue staining - To identify the yellow fever antigen
See Workup for more detail.
Management
No specific treatment exists for yellow fever; however, supportive care is critical. Severely ill patients should be treated in an intensive care setting. The required management consists of the following:
-
Vasoactive medications
-
Fluid resuscitation
-
Ventilator management
-
Treatment of disseminated intravascular coagulation (DIC), hemorrhage, secondary infections, and renal and hepatic dysfunction
Additional supportive care recommendations for patients with yellow fever include the following:
-
A nasogastric or orogastric tube may be required to provide nutritional support
-
Patients with renal failure or refractory acidosis may require dialysis
-
Salicylates should be avoided because of the increased risk of bleeding secondary to platelet dysfunction
Prevention
The currently available yellow fever vaccine confers near lifelong immunity in 95% of patients.
See Treatment and Medication for more detail.
Background
Yellow fever can be transmitted in urban areas by infected Aedes aegypti mosquitoes and in jungle (sylvatic) areas by Haemagogus and Sabethes forest canopy mosquitoes that get the virus from wild primates. The virus is most common during peak rainfall, humidity, and temperature in tropical South America, and late rainy and early dry seasons in Africa. [3] It belongs to the flavivirus family and is one of many causes of viral hemorrhagic fever. Other flaviviral infections include dengue, Japanese encephalitis, West Nile, Zika, and tick-borne encephalitis. These viruses should be considered in cases of CNS infection, hemorrhagic fever, and acute febrile illnesses with joint pain.
Yellow fever virus is shown in the image below. (See Etiology.)
A mosquito-borne disease, yellow fever can manifest on a wide spectrum, ranging from asymptomatic illness to acute-onset viral hepatitis and hemorrhagic fever. (See Clinical and Workup.)
From 1793-1822, yellow fever was one of the most dreaded diseases in US port cities. Yellow fever outbreaks in the United States shaped American history and influenced important national decisions. In the 1780s, yellow fever outbreaks in Philadelphia were responsible for killing one tenth of the city's population. [4]
The disease may have played a part in shaping the decision to move the nation's capital out of Philadelphia. [4] The disease had such an impact on the local economies that, in 1803, Napoleon, with his troops decimated by yellow fever, had few reservations about selling the affected Louisiana and western territories to the US government.
Fascinating accounts document how humankind's struggle with yellow fever has shaped world history. The French effort to develop the Panama Canal was not lost through engineering failures, but by disease. Frenchmen died of yellow fever in alarming numbers, leading to Panama being coined "the white man's graveyard." [5]
In the early 20th century, Carlos Findlay and Walter Reed's discovery of Aedes aegypti as a source of yellow fever transmission led to the eradication of yellow fever in parts of Latin America. Isolation of the virus and later development of the 17D vaccine by Max Theiler helped to eliminate A aegypti and yellow fever from countries in Africa and the Americas during the mid 20th century. [6]
Yellow fever is transmitted by tree-hole breeding mosquitoes (Haemagogus and Aedes species) during the tropical wet season and early dry season. [1] Genomic sequence analyses suggest that yellow fever evolved from other mosquito-borne viruses about 3000 years ago in Africa. It is surmised that the yellow fever virus was introduced to the Americas from West Africa by Dutch slave traders during the 17th century.
The first documented epidemic occurred in the Yucatan Peninsula and spread through the Caribbean basin. This was the result of ship travel and the continued importation of slaves from West Africa. Vessels infested with A aegypti mosquitoes brought yellow fever into New England and several port cities throughout North America.
Large vaccination campaigns and A aegypti control programs have decreased the incidence of yellow fever worldwide. Nonetheless, yellow fever has reemerged across Africa and South America, despite the availability of an effective live-attenuated 17D vaccine. The populations at highest risk for the illness are those in countries that lack the funding and infrastructure to support a widespread vaccination program. (See Epidemiology and Treatment.) [7]
Flaviviruses, including those that cause yellow fever, also have a potential use as biologic weapons. [8]
Transmission
As an arthropod-borne virus (ie, arbovirus), yellow fever is transferred from host to host by contaminated mouthparts of mosquitoes. Different species of the Aedes and Haemagogus genus breed in unique habitats (peridomestically versus within the forest canopy). Consequently, these vectors transmit the virus in 3 ways: (1) between monkeys, (2) from monkeys to humans, and (3) from person to person. [9, 10] This variability has led to 3 types of transmission cycles: sylvatic (jungle), intermediate (savannah), and urban. (See the diagram below.)
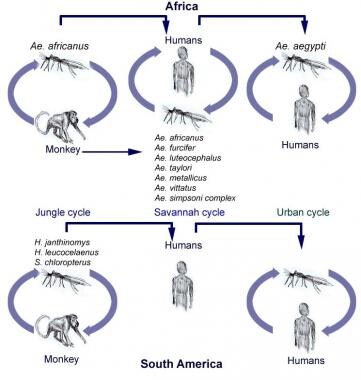 Transmission cycles of yellow fever in Africa and South America. Adapted from Annu Rev Entomol. 2007. 52:209-29.
Transmission cycles of yellow fever in Africa and South America. Adapted from Annu Rev Entomol. 2007. 52:209-29.
 This female Aedes aegypti mosquito is shown after landing on a human host. The A aegypti mosquito is a known transmitter of dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when he or she has been bitten by a female mosquito. Image courtesy of the CDC/World Health Organization (WHO).
This female Aedes aegypti mosquito is shown after landing on a human host. The A aegypti mosquito is a known transmitter of dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when he or she has been bitten by a female mosquito. Image courtesy of the CDC/World Health Organization (WHO).
Sylvatic (jungle) cycle
In tropical rainforests, yellow fever virus is endemic among lower primates. Infected monkeys pass the virus to canopy-dwelling mosquitoes that feed on them. Persons who subsequently enter the forest (often workers, eg, loggers, and travelers) are infected following the bite of an infected mosquito. In Africa, the principal vector of the jungle cycle is A africanus; in South America, Haemagogus species are the primary vector for jungle transmission. Nonhuman primates remain the preferred host and reservoir in this setting.
Intermediate (savannah) cycle
In moist and semihumid areas of Africa, mosquitoes, which breed in the wild and around households, feed primarily on monkeys but will also feed on humans when the opportunity arises. This cycle likely reflects the evolution of yellow fever into an epidemic human disease and is also known as the zone of emergence. It is the most common cycle present in Africa and frequently leads to small-scale outbreaks in villages. However, transmission can potentially lead to large-scale epidemics if an infected individual carries the disease into an urban region. This cycle has not been identified in South America.
Urban cycle
A aegypti is responsible for the transmission of urban yellow fever in Africa and South America (see image below). A aegypti is well adapted as an urban vector: breeding in man-made water containers, feeding primarily on humans, biting multiple individuals during a single blood meal, and transmitting yellow fever efficiently in its saliva. Thus, A aegypti can infect large populations of unvaccinated individuals. Urban outbreaks were rare in South America, yet have been increasingly reported in both South America and Africa, with concern for potential of further spread. [11]
Etiology
Yellow fever virus is a positive-sense, single-stranded, ribonucleic acid (RNA) ̶ enveloped flavivirus with a diameter of about 50-60 nm. The virus is transmitted via the saliva of an infected mosquito. Local replication of the virus takes place in the skin and regional lymph nodes. Viremia and dissemination follow. [3]
The virus gains entrance through receptor-mediated endocytosis. RNA synthesis occurs in the cytoplasm and protein synthesis takes place in the endoplasmic reticulum. Virions are released through the cell membrane. The viral envelope contains a lipid bilayer taken from the infected cell. Virulence factors include the following:
-
Capsid protein C - Facilitates viral binding
-
Membrane protein M - A minor glycoprotein
-
E proteins - Initiate infection, mediate viral entry, and serve as principal targets for host immune response
-
Nonstructural protein 1 (NS1) - May play a role in RNA replication and immune evasion
-
NS2A protein - Involved in RNA replication and packaging
-
NS2B and NS3 - Form a complex and are involved in polyprotein processing and replication of RNA
-
NS5 - Has a major role in RNA replication
The E protein interacts with the cellular receptor, and virions are endocytosed into the dendritic cells. Subsequently, epidermal dendritic cells and lymph channels disseminate virions. After invasion in the host, Kupffer cells (fixed liver macrophages) are infected within 24 hours. [12] Yellow fever is primarily viscerotropic, with the liver being the most affected organ. [13]
The infection quickly disseminates to the kidneys, lymph nodes, spleen, and bone marrow. Renal failure occurs as renal tubules undergo fatty change and eosinophilic degeneration, likely due to direct viral effect, hypotension, and hepatic involvement.
The liver is the most important organ affected in yellow fever. The disease was labeled "yellow" based on the profound jaundice observed in affected individuals. Hepatocellular damage is characterized by lobular steatosis and necrosis, with recent data indicating apoptosis as the primary mechanism of cell death in the liver, corresponding with subsequent formation of Councilman bodies (degenerative eosinophilic hepatocytes). [12, 13]
The kidneys also undergo significant pathologic changes. Albuminuria and renal insufficiency evolve secondary to the prerenal component of yellow fever; consequently, acute tubular necrosis develops in advanced disease. Hemorrhage and erosion of the gastric mucosa lead to hematemesis, popularly known as black vomit. Fatty infiltration of the myocardium, including the conduction system, can lead to myocarditis and arrhythmias.
Central nervous system (CNS) findings can be attributed to cerebral edema and hemorrhages compounded on metabolic disturbances. The bleeding diathesis of this disease is secondary to reduced hepatic synthesis of clotting factors, thrombocytopenia, and platelet dysfunction.
The terminal event of shock can be attributed to a combination of direct parenchymal damage and a systemic inflammatory response. This cytokine storm has been characterized by increased levels of interleukin (IL)-6, IL-1 receptor antagonist, interferon-inducible protein-10, and tumor necrosis factor (TNF)–alpha. Viral antigens are found diffusely in kidneys, myocardium, and hepatocytes. In individuals who survive yellow fever, the recovery is complete, with no residual fibrosis.
Epidemiology
As of June 2024, estimates indicate there are approximately 200,000 cases of yellow fever occurring annually in 47 countries, resulting in 30,000 deaths per year. [9, 14] A 2016 modeling study suggested the disease may infect up to 1.8 million individuals in Africa annually, leading to 180,000 cases and 78,000 deaths. [11] In 2021, there were an estimated 86,509 cases of yellow fever worldwide. [15] World Health Organization (WHO) data suggest that the rate of yellow fever transmission is increasing, especially in sub-Saharan Africa. In addition, the number of US residents traveling to South America and Africa also is increasing.
Occurrence in the United States
Reports of yellow fever in the United States are exceedingly rare, with the last outbreak reported in New Orleans in 1905. It was a rare cause of illness in returning travelers; between 1970 and 2002; 9 cases of yellow fever were reported in unimmunized travelers from the United States and Europe. In these individuals, the disease was acquired in Brazil, Senegal, Venezuela, Ivory Coast, Gambia, and West Africa. Seven of these cases were fatal. [10]
Less fervent mosquito control efforts in the United States have led to the reemergence of Aedes aegypti in the last 30 years. A aegypti has been found in 23 states in the southeastern US. It is still a common mosquito in subtropical regions of southeastern Florida and along the Gulf of Mexico. [9, 16]
After 21st century outbreaks of dengue fever in Hawaii and along the Texas-Mexico border, it has been hypothesized that yellow fever could reemerge in the United States. [17] Virology research has isolated Flaviviridae strains from mosquitoes in eastern Texas, making transmission of urban yellow fever a potential threat for the United States in the future. [18] Failure to sustain vector control campaigns has led to the reinvasion of A aegypti across the Americas, as evidenced by the recent chikungunya virus and Zika virus outbreaks. [11]
International occurrence
As of June 2024, estimates indicate that there are approximately 200,000 cases of yellow fever occurring annually in 47 countries, resulting in 30,000 deaths per year. [9, 14] A modeling study suggested the disease may infect up to 1.8 million individuals in Africa annually, leading to 180,000 cases and 78,000 deaths. [11]
Region of the Americas
In South America, yellow fever transmission historically is linked to outbreaks in the Amazon region carried by Haemagogus mosquitoes. [9, 13, 19] Recent urban outbreaks have been caused by deforestation, [20] migration of unvaccinated population, and the emergence of the Aedes aegypti species.
-
The risk for yellow fever outbreaks is high in the Region of the Americas, with Colombia, Guyana, and Peru reporting cases as of March 19, 2024.
-
In 2023, four countries in the region reported a total of 41 confirmed cases, resulting in 23 deaths.
-
As of the same date in 2024, seven confirmed cases, including four fatalities, have been reported in Colombia, Guyana, and Peru.
-
Brazil reported yellow fever cases in non-human primates but no human cases in 2024.
-
In Colombia, three fatal cases were reported, with cases from Putumayo and Bolivar departments. The individuals had no history of yellow fever vaccination but recovered. [20]
The incidence of yellow fever in South America is lower than in Africa because the infected monkeys in the rain forest canopy do not often come in contact with human populations. Indigenous human populations have immunity as a part of mass immunization campaigns. [21]
Recent outbreaks in urban areas of South America have been due to deforestation (leading to ground-level biting activity of canopy-dwelling vectors), unvaccinated population migration into endemic areas, and the resultant emergence of A aegypti species. Currently, 13 endemic countries within South America have been identified, with Bolivia, Brazil, Columbia, Ecuador, and Peru at greatest risk. [9, 13, 19]
WHO African Region
As of February 25, 2024, 13 countries in the WHO African Region have reported probable and confirmed cases of yellow fever. This includes countries such as Burkina Faso, Cameroon, the Central African Republic, Nigeria, and others. These countries have initiated response planning to address the transmission of yellow fever within their territories. Most cases in Africa occur in regions where Aedes aegypti mosquitoes are prevalent, and the countries at greatest risk are within a specific equatorial band. [22]
-
Transmission primarily occurs in sub-Saharan regions with Aedes aegypti mosquitoes and unvaccinated populations [21]
-
Approximately 20% case-fatality rate in Africa, with infants and children at highest risk [21]
-
Close proximity of vector mosquito populations and unvaccinated humans facilitate transmission in the region, with 34 African countries at risk [21]
WHO reports highlighted yellow fever outbreaks in South Sudan and 13 African countries in 2023-2024, urging increased surveillance efforts to prevent disease transmission through travel and the presence of disease-spreading mosquitoes in neighboring countries.
An outbreak in South Sudan resulted in 64 confirmed cases, including 6 fatalities. The WHO highlighted the risk of urban spread, noting that Aedes mosquitoes in urban areas can amplify outbreaks, citing cases in Cameroon's largest city, Douala. A case-fatality rate of 11% was reported for 2023.
In March 2024, the World Health Organization (WHO) reported that 13 African countries had documented confirmed or probable yellow fever cases since the start of 2023. [20] 2024 The WHO urged an increase in surveillance efforts owing to the potential for disease transmission through travel and the presence of disease-spreading mosquitoes in neighboring countries.
Global expansion and risk in Asia-Pacific
The range of yellow fever is expanding, including areas that previously were thought to be eradicated. Most reported cases are in Africa, but concerns have been raised for potential outbreaks in Asia due to importing viremic travelers, high susceptible populations, and a lack of infrastructure for effective response. [20]
An outbreak of yellow fever in Angola led to 11 imported cases documented in China, marking the first reported cases in Asia and raising concerns about potential introduction and urbanization of the disease in the region.
Factors such as a large community of nonimmune foreign Asian nationals in Angola, coupled with high air travel volumes to susceptible Asian populations and limited responsive infrastructure, pose unprecedented challenges for yellow fever transmission in Asia.
Previous factors, including local vector characteristics and cross-immunity from dengue, have prevented yellow fever from gaining a foothold in the Asia-Pacific region.
While most reported yellow fever cases occur in Africa, [23] there are concerns about outbreaks in the Asia-Pacific region due to importing viremic travelers and susceptible populations. The expansion of the disease's range is notable, with potential risks and challenges for effective response and control measures. [11]
The range of yellow fever continues to expand, now including areas in which it previously was believed to be eradicated (eg, eastern and southern African countries). Outbreaks of yellow fever have not been reported in Asia, but this region remains at risk because of the presence of competent vector mosquitoes and nonhuman primates. [23]
During the recent yellow fever outbreak in Angola, 11 imported cases were documented in China. These were the first yellow fever cases reported in Asia and have led to high levels of concern for potential introduction and urbanization of yellow fever in Asia. The scenario in Angola, which has a large community of nonimmune foreign Asian nationals (home to approximately 100,000 Chinese workers often working in the bush and building roads), coupled with high volumes of air travel to an area conducive to transmission in Asia with a susceptible population of 2 billion people and limited responsive infrastructure, is unprecedented. [23]
Traveler's risk and incidence rates
The risk of acquiring yellow fever depends on travel location, immunization status, season, duration of travel, and activities. The estimated annual incidence of yellow fever cases is approximately 200,000 in 47 countries globally, with 30,000 deaths per year. Accurate reporting is limited due to asymptomatic cases, underreporting, and diagnostic challenges in endemic areas. [20]
(See the image below). [9]
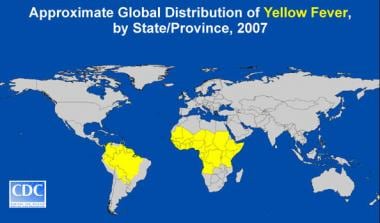 Global distribution of yellow fever. Image courtesy of the Centers for Disease Control and Prevention.
Global distribution of yellow fever. Image courtesy of the Centers for Disease Control and Prevention.
Sex-related demographics
South American cases of yellow fever are sporadic and usually occur in the population exposed to tropical rain forests. Men aged 14-45 years are most often infected through occupational exposure. [19]
In African cases, in which undervaccination of endemic populations has led to higher infection rates in children, yellow fever is slightly more common in males.
Age-related demographics
African cases of yellow fever occur seasonally in villages in contact with semidomestic mosquitoes. In these populations, nonimmunized children are at the highest risk.
Sylvatic disease primarily affects individuals aged 15-45 years who work outdoors in agriculture and forestry. Urban yellow fever and intermediate yellow fever, which occurs primarily in the humid savannas of Africa, affect individuals of all ages. [19]
Prognosis
Yellow fever ranges in severity from a self-limited infection to life-threatening hemorrhagic fever. About 15-25% of affected individuals develop a more severe phase of disease that involves fever, jaundice, and liver and renal failure. Case-fatality rates in South America are reportedly higher than in West Africa. [24] Mortality is a function of patient susceptibility and of the virulence of the infecting strain. [12] In those who become symptomatic but recover, weakness and fatigue can last for months. [21]
The case-fatality rate for yellow fever has been reported at 5%-70%. In recent outbreaks, the fatality rate was approximately 20% among patients with jaundice. The mortality risk in patients who present in the toxic stage of yellow fever is up to 50%. [25]
Death usually follows within 7-10 days of the onset of the toxic phase of yellow fever. [12] Infancy and age older than 50 years are associated with increased severity of illness and lethality. [24]
Unvaccinated travelers entering endemic regions have a greater risk of developing symptomatic disease than natives who have developed significant immunity. [1] An association has been made between recurrent outbreaks in West Africa and a unique strain in that region, suggesting potential strain-specific virulence. [23]
The rare cases of postvaccination neurologic and viscerotropic disease have infrequently led to death. Most individuals diagnosed with yellow fever vaccine–associated neurologic disease (YEL-AND) recover without sequelae; the case-fatality rate has been reported as less than 5%. Even fewer cases of fatal yellow fever vaccine–associated viscerotropic disease (YEL-AVD) have been documented. [24, 26]
Complications
Complications include the following:
-
Liver failure
-
Renal failure
-
Pulmonary edema
-
Myocarditis
-
Secondary bacterial infections
-
Hemorrhage or disseminated intravascular coagulation
-
Encephalitis (rare)
-
Shock or death
Secondary bacterial infections are frequent complications in patients who survive the critical period of illness.
-
Yellow fever virus. Image courtesy of the Centers for Disease Control and Prevention.
-
This female Aedes aegypti mosquito is shown after landing on a human host. The A aegypti mosquito is a known transmitter of dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when he or she has been bitten by a female mosquito. Image courtesy of the CDC/World Health Organization (WHO).
-
Global distribution of yellow fever. Image courtesy of the Centers for Disease Control and Prevention.
-
Transmission cycles of yellow fever in Africa and South America. Adapted from Annu Rev Entomol. 2007. 52:209-29.