Practice Essentials
Colorectal cancer is uncommon in adolescents and young adults; nonetheless, the incidence of colorectal tumors in those age groups is rising. This trend is discordant with that seen in older adults, who are benefiting from screening programs. [1] The incidence of distal colon and rectal tumors is rising at the fastest rate, and rectal tumors are disproportionately represented in the very young age groups. [2]
Most cases of colorectal cancer in adolescents and young adults are sporadic, but several genetic syndromes are associated with these tumors in young patients. The differing biology is suggested by the preponderance of high-grade and mucinous tumors, but the unique oncogenesis is not fully understood. See the images below.
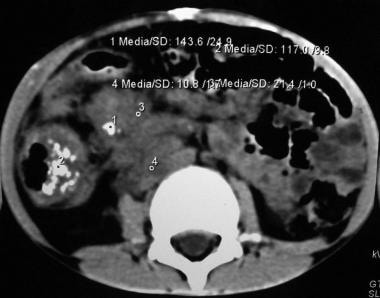 This picture depicts an abdominal CT scan of a 7 year-old boy with a mucinous adenocarcinoma of the ascending colon. Note the thickness and increased vascularity of the colonic wall, as well as irregularities on the serosal surface. This cut also shows severe tumor infiltration of the colonic mesentery surrounding the mesenteric and retroperitoneal vessels.
This picture depicts an abdominal CT scan of a 7 year-old boy with a mucinous adenocarcinoma of the ascending colon. Note the thickness and increased vascularity of the colonic wall, as well as irregularities on the serosal surface. This cut also shows severe tumor infiltration of the colonic mesentery surrounding the mesenteric and retroperitoneal vessels.
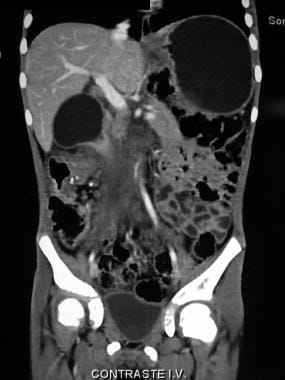 Coronal CT scan demonstrating the profuse tumoral infiltration of the ascending colonic mesentery surrounding mesenteric and portal vessels. Also note the thickness of the colonic hepatic flexure.
Coronal CT scan demonstrating the profuse tumoral infiltration of the ascending colonic mesentery surrounding mesenteric and portal vessels. Also note the thickness of the colonic hepatic flexure.
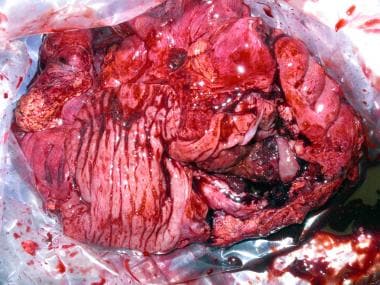 Surgical specimen after right hemicolectomy, including the terminal ileum up to the transverse colon. Mesenteric fat, vessels and lymph nodes were resected en block with the ascending colon. The large intestine has been opened longitudinally. Note the tumor on the right lower quadrant of the image, with severe thickness of the wall, areas of necrosis and hemorrhage, and some stippled calcifications.
Surgical specimen after right hemicolectomy, including the terminal ileum up to the transverse colon. Mesenteric fat, vessels and lymph nodes were resected en block with the ascending colon. The large intestine has been opened longitudinally. Note the tumor on the right lower quadrant of the image, with severe thickness of the wall, areas of necrosis and hemorrhage, and some stippled calcifications.
Multidisciplinary management, including medical, radiation, and surgical oncology teams, is crucial for optimal outcomes.
Surgical treatment of metastatic colorectal cancer offers a chance for cure or prolonged survival.
Patients with peritoneal metastatic disease may benefit from surgical cytoreduction and hyper-thermic intraperitoneal chemotherapy (HIPEC). Best outcomes are documented for those in whom complete cytoreduction is surgically feasible.
Pulmonary metastases are rarely isolated; however, surgical resection is associated with prolonged survival in selected patients. [3]
Background
Colorectal cancer (CRC) is the most common cancer of the gastrointestinal (GI) tract, with over 150,000 new cases diagnosed annually in the United States. In US adults, CRC represents the third most common cancer overall, and the third and fourth leading cause of cancer-related deaths in men and women, respectively. [1] However, CRC is quite rare in children and adolescents, with a reported annual incidence of only one per million persons younger than 20 years in the United States. Only 0.3% of total CRC cases occur in patients younger than 20 years, 2% in those 20-34 years old, and 5.1% in those 35-44 years. [4]
In recent decades, effective screening has reduced the incidence of and mortality from CRC in the older population. However, early-onset CRC is on the rise. The incidence of distal tumors has increased the most, with rectal cancer representing 44% of CRC cases in patients younger than 30 years. [1]
Late diagnosis is not uncommon in younger patients because of the rarity of this entity and the failure to include it within the differential diagnosis for rectal bleeding, chronic abdominal pain, or bowel obstruction in children. In fact, 80-90% of patients younger than 20 years present with stage III/IV disease. [5] Because of advanced stage at diagnosis and often aggressive histology, poor survival is a hallmark of pediatric CRC.
Several inherited polyposis syndromes predispose to CRC, including familial adenomatous polyposis (FAP), Lynch syndrome, MutYH-associated polyposis, juvenile polyposis syndrome, Cowden syndrome, and Peutz-Jeghers syndrome. New syndromes have been defined that give rise to CRC. A rare form of hypermutated microsatellite-stable tumor results from mutation in the POLE gene, which plays a critical role in proofreading to identify and remove mispaired nucleotides during DNA replication. [6] However, the vast majority of CRC cases in adolescents and young adults are sporadic. [7]
Polypoid Disease of the Gastrointestinal Tract
Not all polyposis syndromes are familial. Familial polyposis syndromes are divided into 2 major groups based on the presence of adenomas or hamartomas. The inherited adenomatous polyposis syndromes include familial adenomatous polyposis (FAP) and Turcot syndrome; the familial hamartomatous polyposis syndromes include Peutz-Jeghers syndrome and juvenile polyposis.
Some hamartomas do not appear to have any malignant potential. However, germline mutations and somatic inactivation of STK11, SMAD4, BMPR1A, and PTEN genes in hamartomatous polyposis syndromes create an epithelial environment favorable for neoplastic transformation. [8]
Intestinal polyps, and hamartomas associated with Peutz-Jeghers syndrome, may form a lead point for intussusception. When intussusception occurs in children older than 2 years, the discovery of a specific lead point is not uncommon (22%); however, lead points are only found in 2-8% of children within the usual intussusception age range (6-18 months). When a polyp is demonstrated as a lead point in a patient with intussusception, evaluation for polyposis syndromes may be indicated.
Although juvenile polyps are common in children, adenomas are quite unusual. The latter are considered dysplastic precancerous lesions that are commonly seen in late adulthood. When discovered in children, they suggest one of several types of inherited CRC. [8]
Although the nomenclature is confusing, diffuse juvenile polyposis differs from juvenile polyposis coli. Diffuse juvenile polyposis is a syndrome with multiple polyps spread throughout the GI tract and presents in children aged 6 months to 5 years; in juvenile polyposis coli, the polyps are confined to the rectosigmoid area and are typically found in patients aged 5-15 years. Hamartomatous polyps may also be found in patients with Cowden disease, Cronkhite-Canada syndrome, Bannayan-Riley-Ruvalcaba syndrome, and basal cell nevus syndrome. [9]
Colonic polyposis syndromes
Colonic polyposis syndromes include the following:
-
Nonfamilial polyposis - Isolated juvenile polyps (inflammatory polyps)
-
Familial polyposis - Adenomas (FAP, Gardner syndrome, Turcot syndrome) and hamartomas (juvenile polyposis, Peutz-Jeghers syndrome, Cowden disease, Cronkhite-Canada syndrome)
The lesions can be isolated to the intestine (eg, juvenile, lymphoid, familial adenomatous) or can involve other areas of the body (eg, Peutz-Jeghers syndrome, Gardner syndrome, Turcot syndrome). Most polyps of the GI tract are benign and result from hamartomas of the mucosa or lymphoid hyperplasia of the submucosal layer. However, adenomatous polyps represent a genetic alteration in the mucosa and have substantial malignant potential.
Only the hamartomatous lesions and other nonfamilial lesions are discussed in this section. FAP is presented in detail below, with other cancer-predisposing entities.
Polyps occur in 1% of preschool-aged and school-aged children and are the most frequent cause of rectal bleeding in toddlers and infants aged 2-5 years. Juvenile polyps are the most common (80%), followed by lymphoid polyps (15%). [10]
Isolated juvenile polyps are nonmalignant. By definition, there must be fewer than 5 polyps, limited to the colon, and the child must have no family history of juvenile polyposis. Juvenile polyposis syndromes with malignant potential are classified as follows [11] :
-
Diffuse juvenile polyposis of infancy – Widespread polyposis of the entire GI tract in patients younger than 6 months
-
Diffuse juvenile polyposis – Multiple polyps throughout the GI tract but concentrated in the stomach, distal colon, and rectum; usually occurs in patients aged 6 months to 5 years
-
Juvenile polyposis coli – Multiple polyps confined to the distal colon and rectum in patients aged 5-15 years
Lymphoid polyps (lymphoid nodular hyperplasia)
Lymphoid polyps (present in 15% of patients) are hyperplastic submucosal lymphoid aggregates, most likely due to a nonspecific infection (exposure to bacteria and viruses). Submucosal lymphoid tissue is prominent in children, particularly in the distal ileum (Peyer patches). These non-neoplastic polyps may occur in the rectum, colon, and terminal ileum.
Macroscopically, they appear as firm, round, submucosal nodules that are smooth or lobulated. They are never pedunculated. They often have a volcano-like appearance with mucosal ulceration, which leads to occult blood loss. Histologically, they are hyperplastic lymphoid follicles with a large germinal center covered by colonic mucosa. They develop in young children, with a peak incidence at age 4 years.
Patients present with anemia or, less frequently, with severe rectal bleeding. Barium enema and colonoscopy findings are helpful (in 50% of patients), and biopsy findings confirm the diagnosis.
Surgery is indicated only for uncontrolled bleeding and intussusception that does not respond to enema treatment. Otherwise, expectant measures are adequate because these polyps are benign and spontaneously regress.
Isolated juvenile polyps
Juvenile polyps are mucosal tumors that consist of excessive lamina propria and dilated cystic glands. They usually occur between 2 and 10 years of age and constitute 80% of all polyps in children, with a slight male predominance (3:2). [12]
Juvenile polyps typically present as painless rectal bleeding after defecation. A small percentage debut with prolapse. Prolapsed polyps appear as dark, beefy-red, pedunculated masses, in contrast to the lighter pink mucosal appearance of rectal prolapse. Most prolapsed polyps are erythematous and friable. Abdominal pain is uncommon and is primarily associated with intussusception.
Colonoscopy is the procedure of choice for evaluation of juvenile polyps because those identified in the distal GI tract can be removed at the same time.
Juvenile polyps are solitary in half of patients. The majority are located proximal to the rectosigmoid junction. However, those distally located tend to cause more symptoms. Removal for histologic confirmation is indicated. The typical isolated juvenile polyp with no adenomatous changes has no potential for malignancy and tends not to recur.
Juvenile polyposis syndromes
Juvenile polyposis syndrome (JPS) is a genetic disorder associated with an increased risk of colorectal cancer. JPS is caused by pathogenic germline variants in the SMAD4 or BMPR1A genes.Three quarters of cases are inherited as an autosomal dominant trait; the remainder are sporadic cases in individuals with no family history of the disorder. [13] It is diagnosed in patients with more than 5 polyps at the colon/rectum, multiple polyps throughout the upper and lower GI tract, or any number of lesions with a family history of juvenile polyposis.
According to the St. Mark's Polyposis Registry in London, the cumulative risk of cancer in patients with a juvenile polyposis syndrome is 68% by age 60 years. [14] Consequently, patients with a juvenile polyposis syndrome and their families must receive long-term follow-up. [15]
Some authors advocate prophylactic total colectomy and rectal mucosectomy with an endorectal pull-through (ERPT), [16] whereas others recommend regular screening with colonoscopy and subsequent colectomy if severe dysplasia, rapid polyp formation, or bleeding occurs. [17] Surgery may be offered when the patient has too many polyps to treat endoscopically, or for patients with GI bleeding, anemia, diarrhea, or protein-losing enteropathy. [12]
Diffuse juvenile polyposis of infancy
This entity occurs within the first months of life and is not familial. Patients may present with diarrhea, rectal bleeding, intussusception, prolapse, bowel, protein-losing enteropathy, macrocephaly, clubbing of fingers and toes, and hypotonia. [18]
The entire GI tract is involved. One third of these patients have other congenital abnormalities such as Meckel diverticulum, malrotation, and heart lesions. [19]
Patients require total parenteral nutrition (TPN) and bowel rest, followed by selective resection.
Despite appropriate treatment, this disease is almost universally fatal; only 2 patients have been reported to survive after age 2 years. [18]
Diffuse juvenile polyposis
Diffuse or familial juvenile polyposis was originally identified as isolated or multiple hamartomatous polyps that occur in the colon and rectum of children aged 6 months to 5 years.
Patients present with bright red blood per rectum, anemia, abdominal pain, and rectal prolapse. Diffuse juvenile polyposis is inherited as an autosomal dominant trait [19] ; thus, if a parent has the condition, the risk of having an affected child is 50%.
Hamartomas are malformed colonic mucosa arranged in a bizarre fashion. Typically, hamartomas are not considered premalignant unless they are part of a polyposis syndrome.
Patients with diffuse juvenile polyposis have a 50% lifetime risk of colorectal carcinoma. This may be due to chronic inflammation that produces reactive hyperplasia, which then progresses to dysplasia or adenomatous changes. These polyps often have an ulcerated surface and demonstrate more epithelium with a villous or papillary configuration.
In addition to the epithelial dysplasia that occurs in juvenile polyps, adenomas are also often present. Thus, management is similar to that taken in patients with FAP. Some authors recommend monitoring these patients with an annual complete blood cell (CBC) count (to detect anemia due to GI bleeding), semiannual colonoscopy, and subsequent colectomy if severe dysplasia, bleeding, or rapid polyp formation occurs. Others advocate for prophylactic colectomy.
Associated congenital defects include cleft palate, malrotation, polydactyly, and cranial abnormalities.
Juvenile polyposis coli
A child with 3-10 colonic polyps, any number of polyps in the GI tract outside of the colon, or one polyp and a family history of juvenile polyposis is considered to have the syndrome.
Most patients have 50-100 colorectal polyps; they may also have gastric and small intestinal polyps.
Identifying patients with this syndrome is fundamental because of the high risk for carcinoma (17%) at an early age; the mean age at diagnosis of carcinoma is 35.5 years. [20]
Close long-term surveillance is important. The amount of polyps increases the risk of chronic bleeding, which subsequently leads to iron deficiency anemia, hypoproteinemia, and failure to thrive. [12]
Macroscopically, these polyps resemble isolated juvenile polyps; histologically, however, they have more epithelium with a villous or papillary configuration. Epithelial dysplasia can occur. Adenomas can also be found in conjunction with juvenile polyps. [18]
Peutz-Jeghers syndrome
Peutz-Jeghers syndrome is a cancer-predisposing disorder that is inherited as an autosomal dominant trait. It is characterized by mucocutaneous pigmentation and hamartomatous polyps of the GI tract. Since the tumors tend to recur, extensive bowel resection should be avoided to prevent short bowel syndrome. [21]
In 1921, Peutz reported on the association of intestinal polyps with mucocutaneous pigmented spots of the mouth, hands, and feet. [22] From 1944-1949, in a study of 20 patients, Jeghers defined the 2 main features of the syndrome as melanotic spots on the buccal mucosa and lips (with variable melanin pigmentation on the face and digits) and polyposis of the intestinal tract. The melanotic spots range from brown to black and occur in the rectum, around the mouth, and on the lips, buccal mucosa, feet, nasal mucosa, and conjunctivae. These spots are typically present at puberty. [18]
The polyps most commonly appear in the small intestine (55%), followed by stomach and duodenum (30%) and the colorectal area (15%).
Although adenomas can occur concurrently in the syndrome, these polyps are mostly hamartomas of the muscularis mucosa. They appear as pedunculated lobulated lesions, measuring from a few millimeters to several centimeters. Peutz-Jeghers syndrome is inherited as an autosomal dominant trait, [22] but de novo cases can also develop. It affects all ethnic groups with equal sex distribution [18] ; however, symptoms appear earlier in males (at age 5-10 years) than in females (at age 10-15 years). [22]
GI disturbances become apparent later, usually during early adolescence. Some patients present with an increased frequency of defecation, rectal bleeding, anemia, abdominal pain, vomiting, or recurrent episodes of intussusception. [22] Prolapse of rectal polyps in the first year of life, even in the absence of pigmentation, may indicate Peutz-Jeghers syndrome, at least in the familial cases.
Compared with the general population, patients with Peutz-Jeghers syndrome have a 13-fold increased risk of death due to GI cancer and a 9-fold increased risk for all other cancers. [18] The risk of death due to cancer by age 60 years is 50%, and it reaches 85% by age 70. Adenomatous and carcinomatous changes in the hamartomas have been reported. [23]
Peutz-Jeghers syndrome is closely related to early-onset non-GI malignancies, including breast, ovary, cervix, fallopian tube, thyroid, lung, gallbladder, bile duct, pancreatic, and testicular tumors. [9]
Screening tests to detect all these forms of cancer are recommended in children who present with abdominal pain or occult anemia and melanotic-pigmented spots. An aggressive screening and biopsy program should be undertaken, including an annual examination with CBC count, breast and pelvic examinations (with cervical smears and pelvic ultrasonography) in females, mammography at age 25 years, testicular examination in males, pancreatic ultrasonography, and biennial upper and lower endoscopy.
Extensive intestinal resections are contraindicated because of the recurrent nature of the polyps and the ensuing short-bowel syndrome that may result. Rapid growth, induration, severe dysplasia, villous changes, or polyps larger than 15 mm (which present a much higher risk of malignant transformation) suggest the need for a more aggressive intervention. [18]
Gardner syndrome
Gardner syndrome, which was first described by EJ Gardner and FE Stephens in 1950, is considered a phenotypic variant of FAP in which the adenomatous polyposis is accompanied by extracolonic tumors. [24, 25] The associated extracolonic tumors include desmoid cysts, cysts of the mandible, fibromas, osteomas, and hypertrophy of the retinal pigmented epithelium. Bone tumors are most common (80%), followed by inclusion cysts (35%) and desmoid tumors (18%). Osteomas are most frequently found in the skull and facial bones. Abnormal dentition is common. Periampullary malignancies may develop during the third or fourth decades of life at rates much higher than in the general population. [25]
Like FAP, Gardner syndrome results from a germline mutation in the adenomatous polyposis coli (APC) gene. However, different mutations of the APC gene have been found in Gardner syndrome (polymorphism in exons 13 and 15) compared with FAP. [26]
The natural history and treatment of patients with colonic polyps are the same as in those with FAP. Intestinal polyps have a 100% likelihood of undergoing malignant transformation. [27] Desmoid tumors of the abdominal wall and mesentery occur in 20% of patients with Gardner syndrome, usually appear 6-30 months after surgery for intestinal manifestations, and are the leading cause of death in patients who have undergone colectomy. Desmoid tumors are dense fibroblastic proliferations but can present as dysplasia and even fibrosarcoma.
Treatment is challenging. When these tumors are small and well defined, excision is feasible with a recurrence rate of 10%; however, some are not identified until they become unresectable. Desmoids that involve the small bowel mesentery should be treated according to the symptoms they case, and their growth rate. Sulindac, tamoxifen, or vinblastine and methotrexate are adequate for slow-growing, mildly symptomatic tumors. Aggressive tumors require high-dose tamoxifen, or antisarcoma chemotherapy (doxorubicin and dacarbazine), and possibly radiation therapy. [28]
Oldfield syndrome
Oldfield syndrome is another variant of FAP, in which sebaceous cysts accompany the adenomatous polyposis. Patients present during adolescence with subcutaneous lesions typically located on the extremities, scalp, and face. [29] These patients share the same chromosomal derangements as those with FAP (ie, germline mutations of the APC gene on band 5q21).
Turcot syndrome
This syndrome, also considered a variant of FAP, includes multiple pediatric brain tumors (eg, gliomas, ependymomas) in families that also have an increased risk of polyposis and colon cancer. All individuals with this syndrome develop carcinoma of the colon as young adults. [30] Colonic adenocarcinomas occur in the colonic polyps and in the mucosa between the polyps. Patients may present with chronic bloody diarrhea, hypoproteinemia, weight loss, anemia, malnutrition, bowel obstruction, and intussusception.
Hamilton et al found that families with Turcot syndrome have mutations in APC or HNPCC genes. [31] The type of brain tumor correlates with the mutations: medulloblastomas in APC-related mutations and microsatellite instability in families with glioblastoma multiforme. [18] In patients with a strong family history, diagnostic investigation should begin during the second decade of life and continue annually.
Cronkhite-Canada syndrome
Cronkhite-Canada syndrome is a variant of juvenile polyposis in which the GI polyps are associated with skin hyperpigmentation, alopecia, and nail changes. Hair loss and skin and nail changes may be evident long before GI symptoms appear. The hamartomatous polyps generally involve the entire gastrointestinal tract except for the esophagus; histologically they reveal pseudopolypoid-inflammatory changes. Chronic diarrhea results in malabsorption, hypovitaminosis, hypoproteinemia, and fluid and electrolyte imbalance. Because patients with Cronkhite-Canada syndrome may develop colonic malignancy, close follow-up is recommended. [32]
The etiology of Cronkhite-Canada syndrome remains unknown. Many patients have an autoimmune disease, and reports describe clinical response to immunosuppressive therapy, which further supports an autoimmune etiology. Cases associated with Helicobacter pylori infection have been reported. Isolated reports describe germline variants in the PRKDC gene. [33, 34]
Because of the unknown etiology, treatment for Cronkhite-Canada syndrome remains predominantly symptomatic. Controlled trials have not been possible because of the rarity of the disease. Remissions may occur spontaneously. The primary goal of treatment is to correct fluid, electrolyte, and protein loss, and to regulate stool frequency. These measures help improve the patient's general condition. Most patients need symptomatic treatment for abdominal pain. The most effective treatment is combination therapy composed of systemic corticosteroids together with an elemental diet, and hyperalimentation (nutritional supplements). Other immunosuppressive drugs have also been used (eg, cyclosporine, sirolimus, infliximab). Antibiotics are used to correct intestinal bacterial overgrowth syndrome.
As in all syndromes associated with an increased risk of cancer, patients with Cronkhite-Canada syndrome should receive regular follow-up, to identify malignancies at an earlier stage. A screening schedule similar to the one used for patients with Peutz-Jeghers syndrome is recommended.
Bloom syndrome
Bloom syndrome (congenital telangiectatic erythema) is a rare, recessively inherited disease that is characterized by growth retardation, accelerated aging, immunodeficiency, susceptibility to respiratory and gastrointestinal infections, and a marked predisposition to early development of malignant tumors. [35] The Bloom syndrome gene has been found in chromosome 15. In this regard, genes involved in DNA repair may be considered tumor suppressor genes. Patients with Bloom syndrome appear hypersensitive to various DNA-damaging agents, such as ultraviolet light and irradiation. A generalized DNA repair defect is present, likely a defect in DNA ligation; thus, this process has been encompassed in diseases of DNA repair defects, such as xeroderma pigmentosum, ataxia-telangiectasia, and Fanconi anemia.
The most common early malignancies in individuals with Bloom syndrome are leukemias and non-Hodkin lymphomas. However, second cancers develop in approximately 10% of individuals, with colorectal carcinoma being the most common. [35]
Cowden disease and other PTEN hamartoma syndromes
Cowden disease is part of a spectrum of disorders caused by pathogenic germline variants of the PTEN tumor suppressor gene. Other forms of PTEN hamartoma tumor syndrome (PHTS) include Bannayan-Riley-Ruvalcaba syndrome (previously described as three separate conditions: Riley-Smith syndrome, Bannayan-Zonana syndrome, and Ruvalcaba-Myhre-Smith syndrome). Transmission of PHTS is autosomal dominant, but with extremely variable penetrance. However, PTEN variants can not always be identified in patients with these phenotypes. [36]
Cowden syndrome is characterized by hamartomatous neoplasms of the skin and mucosa (eg, facial trichilemmomas, oral papillomas), bones, central nervous system, eyes, and genitourinary tract. Multinodular goiter, and GI polyps with occasional GI cancer may also occur. Fibrocystic breast disease and esophageal glycogenic acanthosis have been described. [37] These patients have a higher risk of breast and thyroid cancer. Lhermitte-Duclos disease is a variant of Cowden syndrome associated with cerebellar hamartomatous overgrowth.
Treatment is directed toward alleviating pain, bleeding, or obstruction. Polyps should be removed when symptomatic, and screening to detect subsequent development of more polyps is warranted.
Bannayan-Riley-Ruvalcaba syndrome includes developmental abnormalities, macrocephaly, and juvenile polyposis. No cancer predisposition has been reported in these patients. The polyps are removed when symptomatic, and family screening is advised.
Osler-Weber-Rendu disease
Also termed hereditary hemorrhagic telangiectasia (HHT), Osler-Weber-Rendu disease is an autosomal dominant familial disorder characterized by telangiectases and vascular malformations of the skin and mucous membranes and recurrent GI bleeding. It may also affect the brain, lungs, and liver. The lesions are typically noticed in the first few years of life, and 50% of patients have had GI bleeding by the age of 10 years. A family history of the disease is reported in 80% of cases. [38]
Telangiectases are usually present on the lips, oral and nasopharyngeal membranes, tongue, and perlingual areas. They also occur in the colon but are more common in the stomach and small bowel, where they tend to cause significant bleeding. [39]
Although bleeding from mucocutaneous telangiectases and arteriovenous malformations is the principal concern in patients with HHT, colonic neoplasia may also develop. A study by Elinav et al in 24 patients with HHT reported adenocarcinoma of the colon in three patients and multiple colonic polyps in three others. These authors recommend lower GI tract evaluation for neoplasia in all patients with HHT who experience new-onset anemia or GI bleeding, even if the blood loss is thought to be a manifestation of vascular abnormalities from HHT. [40]
Serrated polyposis syndrome
This syndrome, also known as hyperplastic polyposis syndrome, has an unknown molecular etiology and inheritance pattern, arising mostly as a sporadic syndrome; it confers a 35% risk of colorectal cancer. [41]
The colonoscopic criteria for this syndrome include one of the following:
-
At least 5 histologically confirmed serrated polyps proximal to the sigmoid colon, with at least 2 being greater than 1 cm.
-
Any number of serrated polyps proximal to the sigmoid colon, in a patient with a first-degree relative diagnosed with serrated polyposis syndrome.
-
More than 20 serrated polyps scattered throughout the entire colon.
To prevent malignancy in these patients, complete colonoscopic detection and resection of polyps should be performed. In cases in which this is not feasible, surgical resection is recommended. [9]
MutYH-associated polyposis syndrome
The most recently described adenomatous polyposis syndrome, MutYH-associated polyposis (MAP), has an autosomal recessive herency and requires an inherited mutation from each parent for the development of the disease. MAP is caused by a biallelic mutation in the MutYH gene, located in the chromosome 1p33-34, which encodes for a DNA glycosylase responsible for base excision repair; this mutation occurs in 1-2% of the general population. [8]
The polyps found in MAP are typically small tubular or tubulovillous adenomas. In untreated patients, the risk of colorectal cancer is 80% at age 80. In contrast to FAP, the clinical presentation is usually an adult patient with 10 to thousands of polyps, resembling a less severe FAP syndrome that presents during adulthood. [9]
Colorectal carcinoma syndromes
Syndromes associated with CRC include the following:
-
Gardner syndrome - Polyposis, osteomas, and multiple sebaceous cysts
-
Oldfield syndrome - Polyposis and multiple sebaceous cysts
-
Turcot syndrome - Polyposis and brain tumors (gliomas, ependymomas)
-
Peutz-Jeghers syndrome - Colonic polyposis, ovarian tumors, and mucocutaneous pigmentation of lips, oral mucosa, and perioral region
-
Cronkhite-Canada syndrome - GI polyposis, skin hyperpigmentation, alopecia, and nail changes
-
Osler-Weber-Rendu syndrome - Juvenile polyps and hepatic telangiectasia
-
Bloom syndrome - Growth retardation, accelerated aging, immune deficiency, and malignant tumors
-
Cowden syndrome - Hamartomas, GI polyps, breast, thyroid, and GI cancer
-
Serrated polyposis syndrome
-
MutYH-associated polyposis syndrome
The screening schedule for patients with polyposis syndromes and increased risk of malignancy is as follows:
-
Assessment of symptoms related to polyps - Annually
-
Blood count to detect anemia - Annually
-
Breast and pelvic examinations with cervical smears and pelvic ultrasonography in girls - Annually
-
Testicular examination with ultrasonography in boys - Annually
-
Pancreatic ultrasonography - Annually
-
Esophagogastroduodenoscopy and colonoscopy - Biennially
-
Mammography - Recommended at ages 25, 30, 35, and 38 years; biennially until age 50 years; annually thereafter
Most cases of CRC are not inherited as cancer-predisposition syndromes. However, hereditary colorectal cancer is not uncommon; 4-5% of cases are due to Lynch syndrome, and perhaps another 6% are caused by other various hereditary cancer syndromes. [42] Awareness of hereditary colorectal cancer is important to family members, who can direct treatment and cancer surveillance and prevention options.
Familial Adenomatous Polyposis
Familial adenomatous polyposis (FAP) is the most common polyposis syndrome and the second most common inherited colorectal cancer syndrome. [39] It occurs in only 1 in 7000 individuals. This autosomal dominant syndrome is characterized by a mutation in the adenomatous polyposis coli (APC) gene on chromosome 5. Mutations in APC, a tumor suppressor gene that controls tumor initiation, are present in 80-90% of patients with FAP. [28, 43] Approximately 80% of patients have a family history of FAP, while 20% appear to be de novo mutations.
The APC gene encodes a large multifunctional protein product that is involved in a broad spectrum of cellular processes, such as cell cycle regulation, apoptosis, cell adhesion and migration, microtubule assembly, cell fate determination, and chromosomal stability. The main function of APC is to downregulate β-catenin, which inhibits the Wnt signaling pathway. The gene's loss of function leads to increased cellular proliferation.
In persons with a mutated germline APC allele from the affected parent, inactivation (by deletion or mutation) of the normal copy of the APC gene from the unaffected parent completely removes the tumor suppressive function of APC. Inactivation of the second APC allele occurs frequently in the colon and rectum, resulting in the development of numerous adenomatous polyps, beginning in the first and second decades of life. Persons with FAP have an estimated 1 in 471 risk of developing colorectal cancer before the age of 20 years, and their lifetime risk of developing the disease is nearly 100% by 40-50 years of age. [44]
FAP has classic and attenuated forms. In classic FAP, hundreds to thousands of adenomatous polyps tend to concentrate in the distal colon and rectum. Attenuated FAP is characterized by fewer (10-100) polyps, which tend to be located in the proximal large intestine. Age of onset is later than that of classic FAP, with malignant transformation also occurring 10 to 20 years later.
Cancer occurs only rarely before age 20 years in individuals with FAP; however, these cases are usually associated with a severe polyposis phenotype.
Extracolonic manifestations of FAP include congenital hypertrophy of the retinal pigmented epithelium; gastric and duodenal polyps; desmoid tumors and skin lesions, including fibromas, lipomas, and sebaceous cysts; dental anomalies; and osteomas of the jaw and skull. [45]
Prophylactic colectomy is the operation of choice for the management of FAP, although recommendations on the timing or age of colectomy remain unclear. Patients with classic FAP usually undergo surgery between 15 and 25 years of age. [45]
Surgical choices include the following:
-
Total proctocolectomy with ileal pouch anal anastomosis (IPAA)
-
Total abdominal colectomy with ileorectal anastomosis (IRA)
-
Proctocolectomy with ileostomy
Total proctocolectomy with IPAA is an adequate restorative procedure that effectively minimizes the risk of colorectal cancer while maintaining continence. Abdominal colectomy with IRA has also been used; however, 30% of patients who undergo this procedure will develop rectal cancer by age 60 years. [46]
A 1-stage IPAA results in better long-term bowel control but is also associated with an increased incidence of anastomotic leakage, reoperation, and polyp recurrence and should, therefore, be reserved for selected patients. A 2-stage IPAA with diverting ileostomy seems to minimize anastomotic leaks. [45]
For colonic resection, a combination of oral and mechanical bowel preparation is associated with fewer operative complications, including anastomosis leakage, as well as superficial and intra-abdominal infections. [47]
Lynch Syndrome (Hereditary Nonpolyposis Colon Cancer)
Lynch syndrome (LS) is inherited as an autosomal dominant condition marked by increased risks of colorectal cancer (CRC), as well as endometrial, ovarian, gastric, central nervous system, and hematologic malignancies.
LS accounts for 2-4% of all diagnosed cases of CRC. LS-associated CRC is characterized by a relatively young age of onset (45 years) and predominantly right-sided colon cancer, but it has been identified in children aged 9-13 years. The lifetime risk of LS-associated CRC is 52-82%. [19]
In contrast to FAP, which may be considered a disease of tumor initiation, LS may be considered a disease of tumor progression. [48] Benign tumors (ie, adenomas) develop at the same rate in persons with LS as in the general population, but once an adenoma does develop it progresses rapidly. In LS, polyps advance from adenoma to carcinoma in 2-3 years; in contrast, progression to CRC in sporacidic cases takes 8-10 years. Histologically, these colonic tumors are often mucinous, high grade, and poorly differentiated, with large numbers of tumor-infiltrating lymphocytes. [7]
Most cases of LS are caused by germline mutation in one of four DNA mismatch repair (MMR) genes: MLH1, MSH2, MSH6, or PMS2. Rarely, LS results from a deletion in the EPCAM gene that leads to epigenetic inactivation of MSH2. Mutations in MLH1 and MSH2 account for up to 70-90% of defects identified in families with LS, mutations in MSH6 are found in 10-14% of defects, and mutations in PMS2 are found in 15% of defects. LS caused by mutations in MSH2 or MLH1 may present earlier than that caused by mutations in MSH6.
LS becomes manifest when the germline MMR gene mutation is accompanied by a somatic mutation that renders the other allele inactive—the so-called second hit. DNA MMR in the cell then ceases. [42] MMR promotes genomic stability by correcting acid-base and small insertion/deletion loops that form during DNA replication. [19] Without it, mutations involving tumor suppressor genes and oncogenes rapidly accumulate. [48] The resulting changes in sequence length, known as microsatellite instability, are a hallmark of LS. Microsatellite instability is associated with a favorable prognosis and has important implications for therapeutics in CRC.
The American College of Gastroenterology recommends subtotal colectomy with ileorectal anastomosis and postsurgical rectal surveillance for treatment of colon cancer in patients with LS. This procedure may be considered for prophylaxis in selected MMR gene mutation carriers. [49] Carriers of an LS mutation rarely develop CRC in the first 2 decades of life. Prophylactic colectomy may be appropriate for patients whose colons are technically difficult to evaluate by colonoscopy, those who cannot or will not comply with screening recommendations, those with severe psychological distress resulting from fear of developing CRC, or patients with a family history of early-onset CRC. [45] MMR deficiency is not exclusive for LS-associated tumors, as it has been found in 15-20% of sporadic CRC cases. [50]
Familial colorectal cancer type X
There are rare patients who meet the clinical criteria for LS (Amsterdam criteria) but have no mutations in DNA MMR genes. This cancer predisposition syndrome has been termed familial colorectal cancer type X (FCCTX). [51] Like LS, FCCTX is characterized by early-onset CRC in patients and family members from different generations; however, the tumors in these patients do not have MMR deficiency or microsatellite instability. CRC tumors in FCCTX are heterogeneous, consisting mostly of moderately differentiated adenocarcinomas in the rectum and sigmoid colon. The genetic etiology of FCCTX remains uncertain; pathogenic/likely pathogenic variants have been identified in only a minority of patients. [52]
Sporadic Colorectal Carcinoma
CRC in adolescents and young adults may be associated with a familial cancer syndrome. Nevertheless, whereas inherited cancer syndromes are more likely to affect a younger population, most CRC cases in adolescents and young adults are sporadic. Only 22% of adolescents and young adults with CRC have a family history of the disease. [7]
Strong evidence suggests that elevated body mass index in childhood and adolescence increases the risk of early-onset CRC. [53] Other risk factors include prior abdominal radiation for the treatment of childhood malignancies, and inflammatory bowel disease, especially ulcerative colitis. The risk of CRC in ulcerative colitis increases with the extent of the disease and duration of inflammation. CRC risk is estimated at 2%, 8%, and 18% in the first, second, and third decades of active ulcerative colitis, respectively. [54]
By virtue of age alone, all adolescents and young adults with a new diagnosis of CRC should be referred for genetic counseling and testing.
Biology
In patients aged 21 years or younger, high-grade tumors are predominant, with signet-ring cell histology (a feature associated with advanced stage and poorer prognosis) reported in 45% of cases in one cohort. Mucinous histology was reported in 80% of adolescents and young adults and 62% of children with CRC in observational studies. [7]
Microsatellite instability among adolescents and young adults with CRC may be a favorable prognostic indicator.
CRC oncogenesis studies have shown tumors in adolescents and young adults to be more complex than those in patients 45 years and older, and often associated with P53 and PTEN mutations. Interestingly, no PIK3CA mutations have been found in patients younger than 50 years, whereas such mutations are seen in 4-7% of older patients. [55]
Attention is currently directed toward precision medicine. Tumor and genomic mutational analyses are increasingly being used to guide treatment options for patients with malignancies. Particular molecular profiles may identify patients with higher-risk disease, who may benefit from more aggressive treatment or specific targeted therapies. Nicholson et al studied more than 400 patients with CRC and found that loss of Bcl-2 expression is associated with decreased disease-specific and overall survival. This finding could help identify the subset of patients with a more aggressive phenotype and guide therapy choices. [56]
Pathology
Colon cancer is triggered by a series of point mutations and genetic alterations that cause normal cells to transform into adenomas that progressively become dysplastic, resulting in carcinoma foci. [57] These mutations occur in a certain sequence that determines the clinical characteristics of the tumor.
CRC arises from the mucosal surface of the bowel, generally at a site of an adenomatous overgrowth or polyp. The tumor may penetrate the bowel wall and even perforate the serosa into the omental fat, lymph nodes, liver, ovaries, and other loops of bowel. Some lesions cause bowel obstruction. Synchronous lesions may be present, with the same or different histology and stages of development. Carcinoma in situ may occur in one or more polyps. Patients with synchronous primary tumors have the same prognosis as patients with single colon cancers. [58]
The epidermal growth factor receptor (EGFR) is abnormally expressed in CRC cells (72-82%). It promotes cell division, migration, and angiogenesis and inhibits apoptosis. [59] Thus, EGFR plays an important role in the pathogenesis of CRC. Its expression is associated with poor survival and increased risk of metastasis. Italiano et al demonstrated EGFR expression in CRC metastatic cells. [60] Monoclonal antibodies and low molecular weight tyrosine kinase inhibitors may be useful in the therapeutic armamentarium for patients with CRC.
Diagnosis and Management
Surveillance
Colonoscopy with polypectomy of adenomatous polyps results in a 76-90% reduction in the incidence of colon cancer in appropriately screened individuals. Thus, for patients with inherited polyposis syndromes, colonoscopic surveillance is recommended, starting between 10 and 15 years of age.
Proper polypectomy technique decreases the risk of residual polyps and, thus, reduces interval CRC rates. Advanced polypectomy techniques, such as endoscopic mucosal and submucosal resections, have a role in the nonsurgical management of large polyps. [61]
Chemoprevention
Chemopreventive agents may one day inhibit the development of adenomas. Nonsteroidal anti-inflammatory drugs (NSAIDs) have a well-documented effect on shrinking existing adenomas in patients with familial adenomatous polyposis and potentially inhibit their formation. [62] European Society for Medical Oncology guidelines recommend considering aspirin as a cancer prevention measure in patients with Lynch syndrome; the optimal dose has yet to be determined, but one study reported benefit with 600 mg daily. [63]
Clinical presentation
Although colorectal carcinomas constitute only about 1% of all pediatric neoplasms, they are still the most common primary gastrointestinal malignancy. Most cases of pediatric colon cancer occur during late childhood and adolescence. [50] While the gender distribution is equal in adults, the incidence of CRC in children is higher in boys, with a relative ratio of 2:1. [50, 5]
Because of its rarity, CRC is seldom suspected in children and adolescents with abdominal symptoms. Therefore, the diagnosis is often delayed. [50]
Presenting symptoms are nonspecific and include vague abdominal pain, weight loss, nausea, vomiting, anorexia, change in bowel habits (diarrhea or constipation), abdominal mass or distension, rectal bleeding, and/or intestinal obstruction. Left-sided tumors, most common among adolescents and young adults, may cause changes in stool caliber and bowel habits, whereas right-sided tumors are more likely to cause symptoms of anemia. Rectal tumors may lead to blood per rectum and tenesmus.
Advanced stage at presentation is more likely in adolescents and young adults than in older patients. The delay from symptom onset to diagnosis of CRC in adolescents and young adults often exceeds 6 months. A low suspicion rate for malignancy and a lack of screening may contribute to the delay.
Clinical and laboratory investigation
In the absence of rectorrhagia or hematochezia, patients may test positive for occult blood in the stool; however, screening for fecal occult blood has not proven to be of significant diagnostic value for pediatric patients. [32] Anemia may result from blood loss or malnutrition. Liver function abnormalities may be related to metastatic involvement of the liver.
Although fewer than 75% of colon carcinomas in children produce carcinoembryonic antigen (CEA), levels of this protein should be determined. CEA may be a useful tool in identifying recurrent disease after resection, and an increase in CEA levels during follow-up is also related to a higher mortality rate. [64] However, the role of CEA levels in the diagnosis and follow-up of CRC in children is not well established. [5]
International guidelines on CRC in patients younger than 50 years recommend offering multi-gene panel germline genetic testing, with genetic counseling for those with a positive germline finding. At a minimum, the tests should include the following genes: APC, BMPR1A, EPCAM, MLH1, MSH2, MSH6, MUTYH, POLD1, POLE, PMS2, PTEN, SMAD4, STK11, and TP53. [65]
Imaging studies
Patients with clinical manifestations suggesting possible CRC should undergo prompt colonoscopy. [65] Colonoscopy is useful in locating the site of lesions within the large bowel. The entire length of the colon should be evaluated.
Transrectal ultrasonography may help determine the extent of invasion and resectability of rectosigmoid cancer. Intraoperative ultrasonography of the liver may reveal metastases not observed in other imaging studies.
Contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis is used for staging of CRC. [66] The scans define spread to the liver, lungs, or enlarged lymph nodes, as well as pelvic metastases, especially to the ovaries.
CT scanning may be unable to detect intra-abdominal metastases because of lesion size, paucity of intra-abdominal fat, contiguity with the primary tumor, ascites, implant location, and adequacy of bowel opacification. Current CT scanners are able to detect implants as small as 5 mm in diameter. Magnetic resonance imaging (MRI) may further improve detection. [66]
Radioisotope studies should include a bone scan; if the results are positive, bone marrow aspiration and biopsy are indicated to determine spread to the marrow.
Advanced imaging techniques are indicated for specific patient groups. The European Society of Gastrointestinal Endoscopy (ESGE) strongly recommends performing conventional screening with white light colonoscopy in high-risk patients, as well as pancolonic conventional or virtual chromoendoscopy for patients suspected or known to have Lynch syndrome or serrated polyposis syndrome.
The ESGE also recommends that all patients with longstanding colitis undergo periodic pancolonic chromoendoscopies, with either 0.1% methylene blue or 0.1% to 0.5% indigo carmine and targeted biopsies, replacing the common practice of non-targeted 4-quadrant biopsies. [20]
Staging
The American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system is the most commonly used system to evaluate prognosis in colorectal cancer. [67] See the tables below.
Table 1. American Joint Committee on Cancer TNM Staging of Colorectal Cancer (Open Table in a new window)
Primary Tumor (T) |
Nodal Involvement (N) |
Distant Metastasis (M) |
TX: Primary tumor cannot be assessed. |
Nx: Regional lymph nodes cannot be assessed. |
MX: Presence of distant metastasis cannot be assessed. |
T0: No evidence of primary tumor is present. |
N0: No evidence of regional lymph node metastases is present. |
M0: No evidence of distant metastasis is observed. |
Tis: Carcinoma in situ is present. |
N1A: Metastasis in 1 pericolic or perirectal lymph node is present. |
M1A: Distant metastasis is present in a single organ. |
T1: Tumor cells invade the submucosa |
N1B: Metastasis in 2-3 pericolic or perirectal lymph nodes is present. |
M1B: Distant metastasis is present in multiple organs. |
T2: Tumor cells invade the muscularis propria. |
N1C: Metastasis in subserosa, mesentery, or nonperitonealized pericolic or perirectal tissue without lymph node metastasis. |
|
T3: Tumor cells invade the muscularis propria into nonperitonealized pericolic or perirectal tissues. |
N2A: Metastasis in 4-6 pericolic or perirectal lymph nodes. |
|
T4A: Tumor cells perforate the visceral peritoneum. |
N2B: Metastasis in 7 or more pericolic or perirectal lymph nodes is observed. |
|
T4B: Tumor cells directly invade and adhere to other organs and structures. |
|
|
The AJCC prognostic stages are defined in the following table. [67]
Table 2. American Joint Committee on Cancer Prognostic Stages of Colorectal Cancer (Open Table in a new window)
Stage |
Primary Tumor (T) |
Nodal Involvement (N) |
Distant Metastasis (M) |
0 |
Tis |
N0 |
M0 |
I |
T1 |
N0 |
M0 |
|
T2 |
N0 |
M0 |
IIA |
T3 |
N0 |
M0 |
IIB |
T4A |
N0 |
M0 |
IIC |
T4B |
N0 |
M0 |
IIIA |
T1/T2 |
N1A/N1B/N1C |
M0 |
|
T1 |
N2A |
M0 |
IIIB |
T3/T4A |
N1A/N1B/N1C |
M0 |
|
T2/T3 |
N2A |
M0 |
|
T1/T2 |
N2B |
M0 |
IIIC |
T4A |
N2A |
M0 |
|
T3/T4A |
N2B |
M0 |
|
T4B |
N1A/N1B/N1C/N2A/N2B |
M0 |
IVA |
Any T |
Any N |
M1A |
IVB |
Any T |
Any N |
M1B |
Surgical treatment
Surgical resection remains the mainstay of treatment for CRC in adult and adolescent patients, and the sole therapy required for patients with stage I and II disease. [68]
Open colectomy has been the standard of care for the past century. Complete en bloc tumor resection, including the lymphatic basin of the affected segment, has the greatest impact on survival. [50]
Treatment guidelines are based largely on evidence from older adults. Multidisciplinary care is crucial, and prompt referral to centers experienced in the care of adolescents and young adults should be considered for young patients.
Surgical site infection complicates approximately 15% of colectomy procedures. Oral antibiotic bowel preparation before elective colorectal surgery is associated with shorter postoperative length of stay and lower 30-day readmission rates, primarily because of fewer readmissions for infections. [69] Moghadamyeghaneh et al demonstrated that a combination of mechanical and oral antibiotic preparations significantly decreased postoperative morbidity. [47]
Enhanced recovery protocols, which aim to streamline and standardize perioperative care, have demonstrated efficacy in reducing length of stay but have not resulted in reduced readmission rates. These protocols avoid the use of bowel preparation and epidural anesthesia, and encourage early ambulation, early feeding, and early transition to oral analgesia.
Restorative proctocolectomy with ileal pouch anal anastomosis (IPAA) is a viable procedure in pediatric patients, with acceptable morbidity and good long-term results with regard to gastrointestinal function, quality of life, and patient satisfaction. [70, 71] Concerning the type of anastomosis, these authors favor stapled IPAA for prophylactic colectomy, reserving hand-sewn IPAA for patients with neoplasia. The latter is a prudent approach, because earlier dysplasia and colorectal neoplasia are the most important risk factors for developing pouch-related cancer. [70]
An ileal J-pouch is easy to create and delivers acceptable outcomes. However, an S-pouch provides an extra 1-2 cm of length, allowing for tension-free IPAA. [71]
Because children have a long life expectancy, both functional outcomes and control of neoplastic activity of the anal canal and ileal reservoir are of utmost importance. [71]
Best results are obtained with stapled, tension-free anastomosis. Intact tissue rings, good hemostasis, and absence of air leak are imperative. Malnutrition (albumin level, < 3.5 g/dL), neoadjuvant drug toxicity, anemia (hemoglobin level, < 13.5 g/dL), and prolonged high-dose corticosteroid therapy (20 mg of prednisone daily for longer than 3 months) are prognostic factors for perioperative morbidity. Perioperative complications are not uncommon (10%); they include pelvic sepsis, anastomotic leak or stricture, pouchitis, pouch failure, bowel obstruction, and anastomotic stricture. [71]
Laparoscopic resection offers similar postoperative outcomes, pouch function, and long-term quality of life compared with open procedures. [71] However, the learning curve for laparoscopic colectomy remains steep. The need to retract multiple organs, identify complex anatomy, and control large vessels makes the operation difficult. [68]
Patient selection in minimally invasive surgery for colon cancer
Good surgical outcomes depend on careful patient selection. Historic contraindications to a minimally invasive approach include elderly or high-risk patients, multiple previous abdominal operations, technically challenging surgery, and patients with serious comorbidities. All these factors can add a level of difficulty to the performance of an operation, and they are certainly associated with increased morbidity and mortality. But clearly none of these factors are absolute; rather, they are relative contraindications to minimally invasive surgery. The surgeon’s main goal is the performance of a safe and adequate operation. [68]
Laparoscopic surgery has been found to be equivalent to open surgery in terms of oncologic outcome, margins, lymph node sampling, recurrence, and disease-free survival. The ability to obtain an R0 resection with tumor-free margins is a keystone of oncologic colectomy. [68]
Currently, the following are the only 2 absolute contraindications to laparoscopic CRC surgery [68] :
-
A tumor large enough that the extraction site to remove it would be of sufficient size to perform the entire operation.
-
A high-grade large bowel obstruction leading to a reduced intra-abdominal domain. The impaired visibility coupled with the friability of the tissue associated with a cancer makes minimally invasive surgery an ill-advised approach in this setting.
Advantages of minimally invasive surgery in colon cancer
In adults, benefits of minimally invasive surgery include decreased length of stay, smaller incisions, less narcotic use, less blood loss, lower transfusion rates, and improved pulmonary function after surgery. No long-term differences in oncologic outcomes have been demonstrated. [68]
Surgical colon cancer guidelines widely cite consensus for a goal of at least 12 lymph nodes for an adequate staging. There is a positive correlation between the number of lymph nodes examined and survival for patients with stage II and III CRC. No difference has been shown between open and laparoscopic approaches regarding the number of lymph nodes examined, as well as local or distant recurrence rates. [68]
Early reports of a high rate (21%) of port site recurrence raised concerns about minimally invasive surgery. However, larger multicenter, randomized, controlled trials have not shown any significant increase in wound site recurrence. Both laparoscopic and open CRC resections have wound recurrence rates of less than 1%. [68]
Surgery for early-stage disease (non-metastatic)
Radical surgery is the pillar of curative treatment, including en bloc resection of adjacent organs infiltrated by the tumor. A margin of 5 cm of bowel both proximal and distal to the tumor should be removed (although this distal margin may not be feasible with a low-lying rectal tumor). Right hemicolectomy is indicated for tumors proximal to the splenic flexure, whereas left hemicolectomy is carried out for tumors of the descending colon.
Rectal cancer surgery should include total meso-rectal excision (TME) to minimize the risk of local recurrence. It also requires retrieving a minimum number of lymph nodes. The utility of this approach is still uncertain for adolescents and young adults.
Radical resection of low-lying rectal cancers has traditionally required abdominoperineal resection that involves permanent colostomy, which can have significant impact on the quality of life of adolescents and young adults. For very superficial tumors, mucosal resections might be adequate. Trans-anal TME allows for proper oncologic resection without permanent colostomy.
Indications should be made in the context of an integrated treatment strategy established by an interdisciplinary local or reference board. [72]
Treatment of metastatic disease
Metastatic disease occurs in half of patients with CRC. Metastasis can spread through hematogenous, lymphatic, transcoelomic, endoluminal, or contiguity routes, and it may occur in lymph nodes, liver, lung, peritoneum, brain, and bone. Synchronous presentation at diagnosis occurs in 21% of patients and is associated with worse survival than metachronous disease.
Although most cases of metastatic CRC are incurable, combination chemotherapy with improved surgical techniques and radiation therapy plays a role in prolonging survival and reducing cancer-related symptoms in the context of extensive metastatic disease. [3]
The staging work-up for metastatic disease includes CT of the chest, abdomen, and pelvis and consideration of positron emission tomography (PET)/CT for selected patients with indeterminate findings. Routine laboratory work-up, measurement of carcinoembryonic antigen (CEA) level, and determination of RAS and BRAF status of the primary tumor should be performed. Biopsy of metastases may be useful in the setting of first recurrence or in the case of indeterminate imaging findings.
A multidisciplinary evaluation is of critical importance for most patients with metastatic CRC, because the choice and order of therapies differ depending on presentation, number of sites and location of metastases, and potential for surgical resection. [3]
A growing array of agents for targeted therapy of CRC is becoming available. See Colon Cancer. The 5-year relative survival for patients with distant metastasis is currently 15.7 years. [4]
Tumor debulking offers little survival benefit for patients with extensive metastatic disease. [5] However, in the case of a limited burden of cancer spread, surgical resection of metastatic deposits may result in a longer disease-free interval or even a cure in a minority of cases. [7]
Metastasis limited to the liver, lung, or peritoneum may be operable. Resection of peritoneal disease is often performed in conjunction with heated intraperitoneal chemotherapy (HIPEC), although the added value of this therapy compared with surgery alone remains uncertain.
Chemotherapy and radiation therapy
Radiation is often given with or without chemotherapy in the neoadjuvant setting for rectal cancer, to reduce the risk of local recurrence.
Adjuvant chemotherapy may be considered for recurrence risk reduction in stage III or high-risk stage II CRC. Features that indicate a high risk of recurrence in stage II CRC include the following:
-
Peritoneal tumor infiltration (T4)
-
High grade
-
Perforation or obstruction at presentation
-
Lymphovascular or perineural invasion
-
Inadequate lymph node sampling
Combination chemotherapy with a fluoropyrimidine and oxaliplatin for 6 months remains the standard of care, although studies show little difference between 3 and 6 months of therapy in the absence of T4 or heavy lymph node involvement.
After surgery and adjuvant therapy, surveillance monitoring for recurrence includes periodic clinical assessment, CT, colonoscopy, and measurement of CEA levels.
Prognosis
CRC in childhood is associated with a poor prognosis, mainly attributed to delayed diagnosis, greater tumor virulence, and the advanced stage of disease at presentation. Up to 50% of CRC cases that present during childhood are mucinous adenocarcinomas, and 60% have evidence of metastatic disease at presentation. [50]
Signet ring tumors, which are more common in children and adolescents than adults, behave more aggressively and are associated with earlier penetration of the bowel wall and extension along peritoneal surfaces, which suggests more aggressive tumor biology. The mucin absorbs water, swells, and invades tissues, thus promoting spread of malignant cells. Mucin also interferes with the mucopolysaccharide-coating immune recognition of carcinoma cells. [73]
Stage for stage, however, overall and event-free survival seem to be better for pediatric patients. In one study, 5-year survival rates for patients with stage IV CRC were 18.1% for adolescents and young adults versus 6.2% for older adults. [50] Younger patients are more likely to receive systemic therapies and radiation because of sufficient functional reserve and limited comorbidity.
Impact of treatment on fertility
Adhesions following pelvic surgery contribute to infertility risk in women. Pelvic radiation for rectal cancer is likely to cause premature ovarian failure. Options to prevent this complication include pre-radiation laparoscopic ovarian transposition or cryopreservation.
Chemotherapy can also impair reproductive health. Fluorouracil is not likely to cause infertility, but oxaliplatin has been associated with moderate infertility risk in animal studies.
Guidelines
Guidelines on the following aspects of early colorectal cancer have been published:
-
(2024) Management of familial adenomatous polyposis (FAP), MUTYH-associated polyposis, and other rare adenomatous polyposis syndromes (European Hereditary Tumour Group and European Society of Coloproctology) [74]
-
(2023) Management of early-onset colorectal cancer (Associazione Italiana Familiarità Ereditarietà Tumori, the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer, the European Hereditary Tumour Group, and the International Society for Gastrointestinal Hereditary Tumours) [65]
-
(2023) Diagnosis and management of juvenile polyposis syndrome in children and adults (Research Group on Rare and Intractable Diseases [Japan]) [13]
-
(2023) Diagnosis and management of Cowden Syndrome/PTEN hamartoma tumor syndrome in children and adults (Research Group on Rare and Intractable Diseases [Japan]) [37]
-
(2019) Diagnosis, treatment, and follow-up of hereditary colorectal cancer (European Society for Medical Oncology) [63]
-
(2015) Genetic testing and management of hereditary gastrointestinal cancer syndromes (American College of Gastroenterology [49]
-
This picture depicts an abdominal CT scan of a 7 year-old boy with a mucinous adenocarcinoma of the ascending colon. Note the thickness and increased vascularity of the colonic wall, as well as irregularities on the serosal surface. This cut also shows severe tumor infiltration of the colonic mesentery surrounding the mesenteric and retroperitoneal vessels.
-
Coronal CT scan demonstrating the profuse tumoral infiltration of the ascending colonic mesentery surrounding mesenteric and portal vessels. Also note the thickness of the colonic hepatic flexure.
-
Surgical specimen after right hemicolectomy, including the terminal ileum up to the transverse colon. Mesenteric fat, vessels and lymph nodes were resected en block with the ascending colon. The large intestine has been opened longitudinally. Note the tumor on the right lower quadrant of the image, with severe thickness of the wall, areas of necrosis and hemorrhage, and some stippled calcifications.

