Practice Essentials
Upper gastrointestinal bleeding (UGIB) is defined as hemorrhage that emanates proximal to the ligament of Treitz. It is a common and potentially life-threatening condition. Despite evidence of declining incidence and mortality rates, UGIB remains a significant cause of morbidity and mortality, with 100 episodes per 100,000 admissions annually in the United States and mortality rates as high as 14%. [1] Although more than 75% of cases of bleeding cease with supportive measures, a significant percentage of patients require further intervention, which often involves the combined efforts of gastroenterologists, surgeons, and interventional radiologists. Imaging is playing a growing role in the management of acute GI bleeding by localizing the source of bleeding, differentiating the underlying disease processes, and aiding decisions to proceed to endovascular therapies to treat many causes of GI bleeding.
(See the images below.)
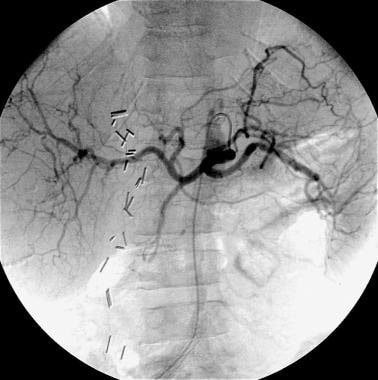 Case 1. Celiac arteriogram obtained in a patient with duodenal ulcer bleeding that was documented endoscopically reveals standard celiac anatomy. No obvious hemorrhage from the gastroduodenal artery is seen.
Case 1. Celiac arteriogram obtained in a patient with duodenal ulcer bleeding that was documented endoscopically reveals standard celiac anatomy. No obvious hemorrhage from the gastroduodenal artery is seen.
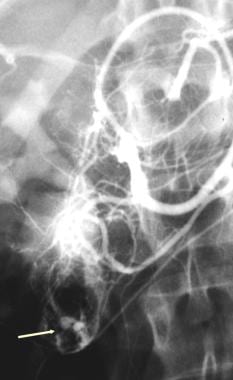 Bleeding from duodenal leiomyoma. Gastroduodenal arteriogram showing a duodenal mass with active contrast extravasation (arrow).
Bleeding from duodenal leiomyoma. Gastroduodenal arteriogram showing a duodenal mass with active contrast extravasation (arrow).
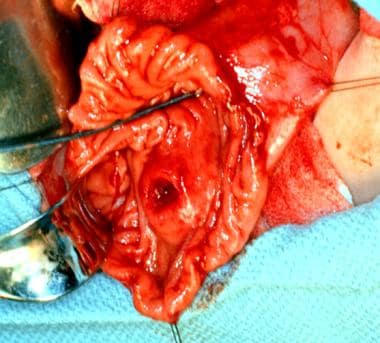 Duodenal resected specimen showing a submucosal mass with a central ulceration (arrow) (same patient as previous image).
Duodenal resected specimen showing a submucosal mass with a central ulceration (arrow) (same patient as previous image).
Clinically, UGIB often causes hematemesis (vomiting of blood) or melena (passage of stools rendered black and tarry by the presence of altered blood). The color of the vomitus depends on its contact time with the hydrochloric acid of the stomach. If vomiting occurs early after the onset of bleeding, it appears red; with delayed vomiting, it is dark red, brown, or black. Coffee-ground emesis results from precipitation of blood clots in the vomitus.
Radiography
Plain radiographs of the abdomen are not usually helpful in the diagnosis of acute upper gastrointestinal bleeding (UGIB). The pathophysiology of acute UGIB is often mucosal erosion with subsequent hemorrhage, which is not detected with plain radiographs. Occasionally, free air under the diaphragm is seen in cases of perforated viscous, and this may be accompanied by UGIB. Other etiologies, such as upper GI masses (which usually result in chronic, not acute, UGIB), aneurysms with calcifications, and ascites suggestive of portal hypertension, may be seen on radiographs. [7]
The radiographic findings, as outlined above, are usually nonspecific. Calcifications associated with aneurysms, in the aorta or branch vessels, are reliable but rare findings regarding a source of upper gastrointestinal bleeding.
Computed Tomography
Usually, abdominal CT is not used in the evaluation of acute upper gastrointestinal bleeding from arterial sources, although it has been helpful in some series. However, in the detection of UGIB from pseudoaneurysms of the mesenteric vessels, branches of the celiac axis, or aortoenteric fistulas, it is the study of choice. In addition, in the evaluation of masses of the upper GI system or liver tumors that may be contributing to hemobilia, CT is an excellent modality. Occasionally, hemorrhage into the peritoneum can be detected on CT scans. [8, 9]
Small pseudoaneurysms, small bowel tumors, and small biliary tumors can easily be missed by CT. In the catastrophic situation of aortoenteric fistula, CT may be helpful in detecting an early leak. The usual drawbacks of upper abdominal CT for the evaluation of subtle lesions also apply to the use of this modality in the evaluation of upper gastrointestinal bleeding. These include underopacification of bowel loops, suboptimal visualization of the biliary system and small visceral aneurysms, and difficulty in evaluating the esophagus.
(See the images below.)
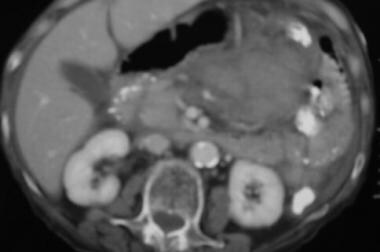
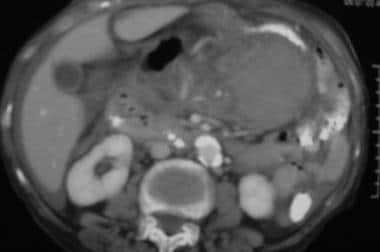 Case 4. CT scan obtained in a 45-year-old man with acute hematemesis reveals a left upper quadrant acute mesenteric and lesser sac hemorrhage.
Case 4. CT scan obtained in a 45-year-old man with acute hematemesis reveals a left upper quadrant acute mesenteric and lesser sac hemorrhage.
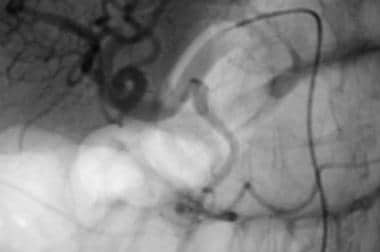 Case 4. Image obtained with a selective common hepatic artery injection reveals a pseudoaneurysm in the right gastric artery, which arises from the proper hepatic artery. The pseudoaneurysm has the appearance of a double density that overlaps the origin of the gastroduodenal artery in this projection.
Case 4. Image obtained with a selective common hepatic artery injection reveals a pseudoaneurysm in the right gastric artery, which arises from the proper hepatic artery. The pseudoaneurysm has the appearance of a double density that overlaps the origin of the gastroduodenal artery in this projection.
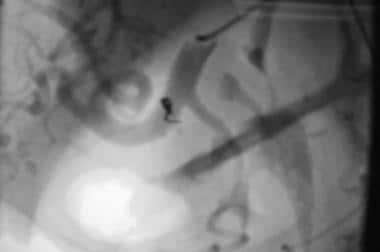 Case 4. Image in a left anterior oblique projection compared with the orientation of the diagnostic angiogram depicts selective embolization of the pseudoaneurysm of the right gastric artery with coils. The patient had no further bleeding.
Case 4. Image in a left anterior oblique projection compared with the orientation of the diagnostic angiogram depicts selective embolization of the pseudoaneurysm of the right gastric artery with coils. The patient had no further bleeding.
In the setting of portal hypertension, the presence of varices and the patency of the portal and splenic veins can be evaluated prior to a transjugular intrahepatic portosystemic shunt (TIPS) procedure or splenic artery embolization.
CT angiography
On CT angiography (CTA), the critical imaging finding of GI bleeding is active extravasation of IV contrast into the bowel lumen. This can be diagnosed with CTA when an intraluminal focus of high attenuation (>90 HU) is seen on arterial phase images (“contrast blush”) that is not present on noncontrast images. On portal venous phase images, this extravasation should change in appearance and generally moves distally within the bowel lumen. The extravasation itself can have a variety of imaging appearances depending on the underlying physiology, including a linear “jet” of contrast, “cloudlike” morphology, circular configuration, or a contrast fluid level. [6]
On CTA, fluid distention of bowel loops may dilute contrast extravasation, potentially causing a false-negative result. High-density material within or near the bowel lumen, including surgical clips, ingested material, and fecaliths, can be mistaken for hemorrhage without a noncontrast scan. Cone-beam artefacts can also lead to the false appearance of high density within the bowel lumen. [6]
Magnetic Resonance Imaging
MRI has a limited role in the evaluation of acute upper gastrointestinal bleeding (UGIB) from arterial sources. In the setting of aneurysms and pseudoaneurysm, magnetic resonance angiography (MRA) may be helpful in depicting the vascular abnormalities. With magnetic resonance cholangiography, the depiction of subtle biliary abnormalities may be helpful in cases of hemobilia. MRI is comparable to CT in the evaluation of masses that cause UGIB.
Similar to CT, MRI has no real role in the assessment of acute upper gastrointestinal bleeding. It may be helpful in depicting small visceral pseudoaneurysms or masses, but a normal MRI finding is often only a starting point for further investigation.
Nuclear Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines recommend gastrointestinal bleeding scintigraphy (GIBS) be used to determine whether the bleeding is active, to localize the bleeding site, and to approximate the bleeding volume for prognostic purposes. Although GIBS can identify overt upper gastrointestinal bleeding, its primary indication is for overt mid or lower gastrointestinal bleeding, GIBS is also indicated to help identify the source of obscure overt gastrointestinal bleeding. Among some of the other common clinical indications for GIBS are stratifying risk in patients with gastrointestinal bleeding, directing timely diagnostic angiography, and assisting in plans for surgical or other interventional procedures. [10]
Radionuclide imaging for GI bleeding is generally performed with technetium-99m tagged red blood cells (RBCs), with initial injection of radiotracer and subsequent gamma camera imaging. GI bleeding can be diagnosed when radiotracer activity is visualized outside of normal areas of blood pool, which either focally intensifies or moves over time in an antegrade or retrograde fashion. Scintigraphy can assess for bleeding over a prolonged period of time and can detect both arterial and venous hemorrhage. [6]
Advantages of radionnuclinde imaging are that it enables continuous monitoring of the entire gastrointestinal tract for up to 24 hours. The ability to perform continuous imaging increases the likelihood of detection of intermittent bleeding over other techniques that are limited a single time point or periodic sampling. GIBS does not require any patient preparation, can be performed with standard nuclear medicine instrumentation, and is well tolerated even in patients who are acutely ill. [10]
However, because of the prolonged imaging time, these studies are not ideal for patients who are clinically unstable. In addition, radionuclide imaging may have limited availability in the acute care setting, particularly overnight. [6]
Angiography
Angiography is often the next step if medical management or endoscopy fails to control upper gastrointestinal bleeding (UGIB). Angiography is minimally invasive; it often allows precise localization of bleeding; and it enables the use of therapeutic options, which include embolization or vasopressin infusion. A hemorrhage rate of 0.5-1.0 mL/min is required before it can be visualized with angiography. The detection of bleeding may be enhanced with carbon dioxide as an arterial contrast agent because of the low viscosity of the gas. [11, 12, 13, 14]
Endoscopic evaluation is critical in providing information for subsequent angiography. [15] For example, if variceal bleeding is suspected instead of arterial bleeding, endoscopy can be used to guide subsequent therapy in favor of a transjugular intrahepatic portosystemic shunt (TIPS) procedure. [16] In cases such as hemorrhagic gastritis (shown in the images below), which is an important cause of UGIB caused by physiologic stress, the endoscopic diagnosis can guide subsequent vasopressin infusion. If a mass or recurrent bleeding from a suspected ulcer in the duodenum is present, the gastroduodenal artery can be embolized.
(See the images below.)
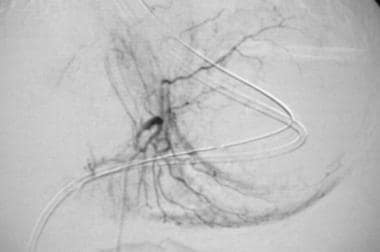 Case 2. Selective left gastric arteriogram obtained in a patient with cirrhosis, recent upper GI bleeding, and esophageal ulcers, with diffuse hemorrhagic gastritis as demonstrated at recent endoscopy.
Case 2. Selective left gastric arteriogram obtained in a patient with cirrhosis, recent upper GI bleeding, and esophageal ulcers, with diffuse hemorrhagic gastritis as demonstrated at recent endoscopy.
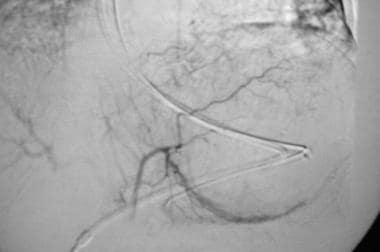 Case 2. Because the patient was known to have diffuse hemorrhagic gastritis, as assessed with endoscopy, vasopressin infusion was begun at 0.2 U/min. Follow-up angiogram reveals a decrease in vascularity to the stomach. Vasopressin was continued overnight, with the patient in the ICU. The patient weaned from the medication over the following 24 hours without a recurrence of bleeding.
Case 2. Because the patient was known to have diffuse hemorrhagic gastritis, as assessed with endoscopy, vasopressin infusion was begun at 0.2 U/min. Follow-up angiogram reveals a decrease in vascularity to the stomach. Vasopressin was continued overnight, with the patient in the ICU. The patient weaned from the medication over the following 24 hours without a recurrence of bleeding.
Angiographic evaluation of UGIB is usually performed via common femoral artery access achieved with the Seldinger technique. A catheter is directed into the celiac artery and superior mesenteric artery for angiography. Prior diagnostic examinations such as endoscopy or CT can be used to guide subsequent catheterization.
Acute arterial bleeding is seen as the extravasation of contrast medium of arterial opacity at the bleeding site. The extravasating contrast agent frequently flows toward the dependent part of the viscous, creating the pseudovein appearance. If the bleeding is demonstrated on the celiac or superior mesenteric angiogram, a more selective injection of the extravasating artery (superselective catheterization) is performed for confirmation of the bleeding and embolization. If contrast agent extravasation is not seen with the selective injections, superselective catheterization of the gastroduodenal, left gastric, and splenic arteries is performed.
Angiography is insensitive in the detection of venous bleeding, such as variceal hemorrhage from portal hypertension. Clinical suspicion and endoscopic findings are helpful in evaluating variceal bleeds. However, angiography can be helpful in the detection of as much as 50% of occult UGIB.
(See the images below.)
 Bleeding from duodenal leiomyoma. Gastroduodenal arteriogram showing a duodenal mass with active contrast extravasation (arrow).
Bleeding from duodenal leiomyoma. Gastroduodenal arteriogram showing a duodenal mass with active contrast extravasation (arrow).
 Duodenal resected specimen showing a submucosal mass with a central ulceration (arrow) (same patient as previous image).
Duodenal resected specimen showing a submucosal mass with a central ulceration (arrow) (same patient as previous image).
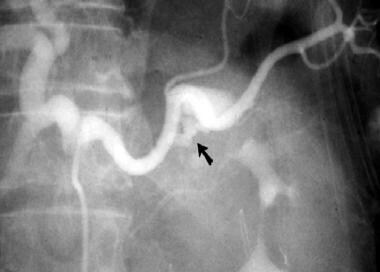 Hemosuccus pancreaticus in patient with upper GI hemorrhage. Splenic artery aneurysm with bleeding into the pancreatic duct (arrow).
Hemosuccus pancreaticus in patient with upper GI hemorrhage. Splenic artery aneurysm with bleeding into the pancreatic duct (arrow).
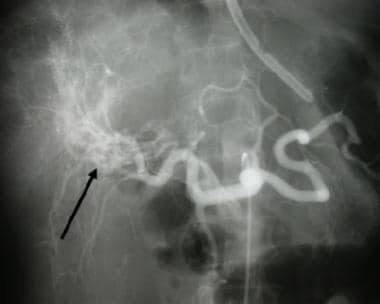 Variceal bleeding in a patient with hepatocellular carcinoma invading the portal vein. Threads and streaks sign (arrow) indicating portal vein invasion by hepatoma.
Variceal bleeding in a patient with hepatocellular carcinoma invading the portal vein. Threads and streaks sign (arrow) indicating portal vein invasion by hepatoma.
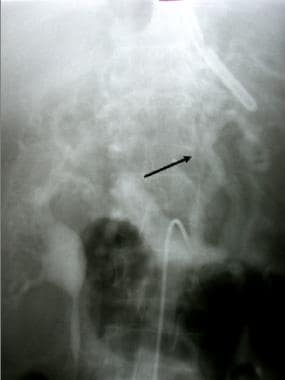 Portal venous phase of a superior mesenteric angiogram showing portal vein occlusion and gastroesophageal varices (arrow) (same patient as previous image).
Portal venous phase of a superior mesenteric angiogram showing portal vein occlusion and gastroesophageal varices (arrow) (same patient as previous image).
Degree of confidence
The degree of confidence in the angiographic diagnosis of upper gastrointestinal bleeding is high. Arteriography can be used to accurately identify 90% of acute UGIB. It is also useful in depicting occult bleeding in 50% of patients. However, a bleeding rate of 0.5-1.0 mL/min is required for the angiographic depiction of hemorrhage. Venous bleeding (eg, from variceal bleeding) is difficult to detect with angiography.
Bowel motion, prior barium examinations, and abnormal staining from inflammation or breathing artifact can hinder angiographic depiction of bleeding. Multiple superselective injections may be required for a confident diagnosis. A bleeding rate of 0.5-1.0 mL/min is required to visualize angiographic hemorrhage; otherwise, a false-negative angiogram results. Alternatively, if the bleeding has stopped at the time of study, the clinical parameters may be helpful in this distinction. Venous hemorrhage from portal hypertension might be another cause of false-negative findings.
-
Case 1. Celiac arteriogram obtained in a patient with duodenal ulcer bleeding that was documented endoscopically reveals standard celiac anatomy. No obvious hemorrhage from the gastroduodenal artery is seen.
-
Case 1. Image depicts selective catheterization of gastroduodenal artery (GDA). Attenuation of the GDA is secondary to recent hemorrhage and vasospasm.
-
Case 1. Image obtained in a patient with recurrent duodenal ulcer bleeding depicts selective embolization of the gastroduodenal artery with coils. When prophylactic embolization is performed in duodenal bleeding, one may embolize the proximal gastroepiploic artery with coils to protect the stomach, and then embolize the branches of the gastroduodenal artery with either Ivalon or Gelfoam.
-
Case 1. Arterial-phase image obtained with selective injection of the superior mesenteric artery shows no contribution of the inferior pancreaticoduodenal arcade to the duodenal ulcer; thus, no embolization was performed. Injecting the appropriate collaterals in upper GI bleeding is important to prevent further hemorrhage.
-
Case 1. Late-arterial phase superior mesenteric angiogram shows no contribution of the superior mesenteric artery to the duodenal ulcer hemorrhage.
-
Case 2. Selective left gastric arteriogram obtained in a patient with cirrhosis, recent upper GI bleeding, and esophageal ulcers, with diffuse hemorrhagic gastritis as demonstrated at recent endoscopy.
-
Case 2. Because the patient was known to have diffuse hemorrhagic gastritis, as assessed with endoscopy, vasopressin infusion was begun at 0.2 U/min. Follow-up angiogram reveals a decrease in vascularity to the stomach. Vasopressin was continued overnight, with the patient in the ICU. The patient weaned from the medication over the following 24 hours without a recurrence of bleeding.
-
Case 3. Selective celiac arteriogram reveals active bleeding in the stomach from a branch of the left gastric artery along the greater curvature of the stomach.
-
Case 3. Image obtained with a selective injection of the feeding vessel of the left gastric artery shows extravasation of contrast material into the dependent portion of the stomach; this finding is the pseudovein sign.
-
Case 3. Image depicts superselective angiography and embolization of branch vessel of the left gastric artery with a platinum coil using a 3F microcatheter. A persistent pseudovein sign is shown.
-
Case 3. Image obtained with a selective gastroduodenal artery injection with filling of the right gastroepiploic artery shows that small branches fill the bleeding vessel along the greater curvature of the stomach.
-
Case 3. Embolization of the right gastroepiploic artery with Gelfoam and a single embolization coil.
-
Case 3. Image obtained with a final superior mesenteric arterial injection to evaluate the collateral contribution to bleeding.
-
Case 4. CT scan obtained in a 45-year-old man with acute hematemesis reveals a left upper quadrant acute mesenteric and lesser sac hemorrhage.
-
Case 4. CT image caudal to previous image reveals acute hemorrhage.
-
Case 4. Image obtained with a selective common hepatic artery injection reveals a pseudoaneurysm in the right gastric artery, which arises from the proper hepatic artery. The pseudoaneurysm has the appearance of a double density that overlaps the origin of the gastroduodenal artery in this projection.
-
Case 4. Image in a left anterior oblique projection compared with the orientation of the diagnostic angiogram depicts selective embolization of the pseudoaneurysm of the right gastric artery with coils. The patient had no further bleeding.
-
Case 5. Portal venogram obtained in a patient with gastric variceal bleeding before the placement of a transjugular intrahepatic portosystemic shunt shows large gastric varices.
-
Case 5. Venogram obtained after the placement of a 12-mm transjugular intrahepatic portosystemic shunt and selective coil embolization of gastric varices.
-
Bleeding from duodenal leiomyoma. Gastroduodenal arteriogram showing a duodenal mass with active contrast extravasation (arrow).
-
Duodenal resected specimen showing a submucosal mass with a central ulceration (arrow) (same patient as previous image).
-
Gastroduodenal arteriogram with a pancreatic pseudoaneurysm (arrow).
-
Control of upper GI hemorrhage by coil occlusion of the pancreatic artery pseudoaneurysm (arrow) (same patient as previous image).
-
Hemosuccus pancreaticus in patient with upper GI hemorrhage. Splenic artery aneurysm with bleeding into the pancreatic duct (arrow).
-
Variceal bleeding in a patient with hepatocellular carcinoma invading the portal vein. Threads and streaks sign (arrow) indicating portal vein invasion by hepatoma.
-
Portal venous phase of a superior mesenteric angiogram showing portal vein occlusion and gastroesophageal varices (arrow) (same patient as previous image).