Practice Essentials
Hypertrophic pyloric stenosis (HPS) is commonly encountered in pediatric practice and is the most common gastrointestinal disease in infants (incidence rate, 1.56 per 1000 live births). [1] HPS usually presents at 2-12 weeks after birth, often in a previously healthy infant, with peak onset at week 5. The typical infant presents with nonbilious projectile vomiting and dehydration (with hypochloremic hypokalemic metabolic alkalosis) if the diagnosis is delayed. The presentation age of preterm infants is defined less clearly. Studies suggest that preterm infants may present with HPS symptoms at a later chronological age than term infants. [2, 3, 4, 5, 6, 7]
Stark et al reported a series of 2466 newborns with HPS, 208 (8.43%) of whom were premature. Chronological age at presentation was found generally to increase as gestational age decreased. Preterm birth was associated with longer interval from birth to presentation with HPS (median [IQR] days of 40 [30-56] vs. 33 [26–45] in full-term infants; P< 0.001). [7] HPS occurs in infants usually in the first 2 months of life and is rare in infants older than 6 months. [8, 9]
This condition accounts for one third of nonbilious vomiting occurrences in infants and is the most common reason for laparotomy before age 1 year. [10] A striking male preponderance is seen, with a male-to-female ratio of 4-6:1.
Radiography
Abdominal radiographs may show a fluid-filled or air-distended stomach, suggesting the presence of gastric outlet obstruction. A markedly dilated stomach with exaggerated incisura (caterpillar sign) may be seen, which represents increased gastric peristalsis in these patients (see the image below). [32] This peristaltic wave may be seen on visual inspection of the abdomen. [23] If the patient has recently vomited or has a nasogastric tube in place, the stomach is decompressed and the radiographic findings are normal.
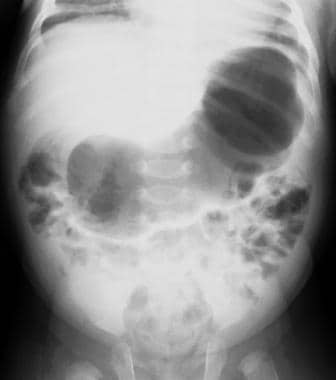 Supine radiograph in an infant with vomiting demonstrates the caterpillar sign of active gastric hyperperistalsis.
Supine radiograph in an infant with vomiting demonstrates the caterpillar sign of active gastric hyperperistalsis.
A UGI study was once considered the test of choice for hypertrophic pyloric stenosis. Findings on UGI studies include the following:
-
Delayed gastric emptying (if severe, this may prevent barium from passing into the pylorus and severely limit the study)
-
Cephalic orientation of the pylorus
-
Shouldering (ie, filling defect at the antrum created by prolapse of the hypertrophic muscle)
-
Mushroom or umbrella sign (ie, thickened muscle that indents the duodenal bulb; the name refers to the impression made by the hypertrophic pylorus on the duodenum)
-
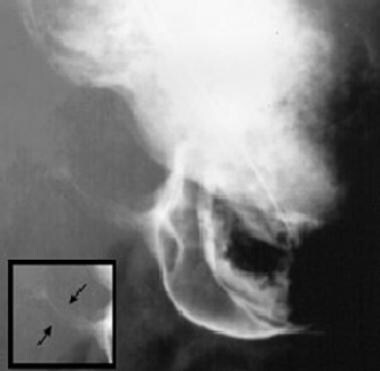
String sign (ie, barium passing through the narrowed channel, creating a single, markedly attenuated, and elongated track) (see the following image)
-
Pyloric teat (ie, outpouching created by distortion of the lesser curve by the hypertrophied muscle)
-
Retained secretions and retrograde peristalsis
Plain film radiography provides a low degree of confidence in making the diagnosis of, or in ruling out, hypertrophic pyloric stenosis. A UGI study has high sensitivity (>90%) and low specificity.
High intestinal obstruction can be seen with midgut volvulus, duodenal obstruction (from stenosis, duodenal web, annular pancreas), gastric outlet obstruction caused by focal foveolar hyperplasia, and eosinophilic gastroenteritis, among others. False-negative radiographs can be seen in a child who has recently vomited.
Ultrasonography
Ultrasonography is important in the diagnosis of hypertrophic pyloric stenosis and has likely contributed to the changing face of the disease, because this modality results in earlier diagnosis and treatment due to the accessibility and accuracy of ultrasonography. [21] This modality is the method of choice for both the diagnosis and exclusion of hypertrophic pyloric stenosis, because ultrasonography has a sensitivity and specificity of approximately 100%. [27, 28, 31, 33] Thus, ultrasonography is also recommended in patients whose disease is clinically suspected but in whom the pyloric olive cannot be felt. [19, 27, 34, 35, 36, 37]
In a study by Leaphart et al, ultrasonography confirmed hypertrophic pyloric stenosis when the pyloric muscle thickness (MT) was greater than 4 mm and the pyloric channel length (CL) was greater than 15 mm. [38] The investigators studied the diagnostic criteria for this disease in newborns younger than 21 days and found that ultrasonographic measurement of hypertrophic pyloric stenosis was significantly decreased in younger patients (MT, 3.7 +/- 0.65 mm; CL, 16.9 +/- 2.8 mm) versus older newborns (MT, 4.6 +/- 0.82 mm; CL, 18.2 +/- 3.4 mm). Of important note, the mean ultrasonographic measurement for young newborns with hypertrophic pyloric stenosis typically fell within the range currently defined as normal or borderline. [38] A linear relationship existed between pyloric MT and CL and patient age, suggesting that 3.5 mm MT be considered the cutoff in younger patients. [38]
The ACR has noted the following in its recommendations for the diagnosis of HPS [30] :
-
US of the abdomen (UGI tract) is usually appropriate for the initial imaging of an infant older than 2 wk and up to 3 mo with new-onset nonbilious vomiting (suspected HPS).
-
US is highly accurate for diagnosing HPS, with sensitivity, specificity, and accuracy of 100%.
-
US allows real-time imaging of the pyloric muscle and channel.
-
The diagnosis of HPS is based on imaging of a constant elongated, thick-walled pylorus with no passage of gastric content and is supported by measurements of pyloric channel length and muscle thickness.
-
Muscle thickness ≥4 mm with a length >18 mm is considered positive for HPS, but measurements between 3 and 4 mm may also be positive, particularly in the premature or younger neonate.
-
Muscle thickness measurement may be obtained on transverse or longitudinal views of the pylorus. In some patients, there is overlap of these measurements, most notably between patients with pylorospasm and patients with evolving HPS. Pylorospasm is considered to be the most common cause of gastric outlet obstruction in this age group and is treated conservatively.
In a retrospective study by Piotto et al of 607 patients (615 scans) who underwent ultrasonography testing for HPS, muscle thickness in the normal group was less than 2 mm, versus 2-5 mm in infants with HPS; pyloric canal length in the normal group was less than 5 mm, versus 10-24 mm in those with HPS; and transverse diameters ranged from 6 to 11 mm in the normal group, versus 8-16 mm in the HPS patients. The authors suggested that the commonly used measurement of 16 mm is too long for canal length and should be reduced to 10 mm. They also noted that muscle thickness in infants with HPS can be as low as 2 mm, versus the current standard of 3 mm. [39]
A study by Park et al of infants (< 90 days) who were brought to the ED for vomiting and underwent point-of-care ultrasound (POCUS) showed that POCUS had a sensitivity of 96.6% and a specificity of 94.0% for HPS. [40]
Technique
Ultrasonography is performed with a 7.5- to 13.5-MHz linear transducer in the supine child. Transverse images at the epigastrium identify the pylorus to the left of the gallbladder and anteromedial to the right kidney (see the image below). A distended stomach, however, displaces and distorts the pylorus and may require the placement of a nasogastric tube to withdraw the stomach's contents. A gastric aspirate of more than 5 mL in a baby who has been without oral intake (NPO) for several hours indicates gastric outlet obstruction. Right posterior oblique positioning and scanning from a posterior approach may help improve visualization of the pylorus. [41]
Ultrasonographic signs of hypertrophic pyloric stenosis, originally described in 1977 [42] and further defined, are as follows:
-
An MT (serosa to mucosa) greater than 3 mm (a correlation between MT and the patient's age exists; the most reliable ultrasonographic sign is an MT greater than 3 mm. Because this measurement can be increased falsely with off-axis imaging, attention to technique is important.)
-
Pyloric channel length greater than 17 mm
-
Pyloric thickness (serosa to serosa) of 15 mm or greater
-
Failure of the channel to open during a minimum of 15 minutes of scanning
-
Retrograde or hyperperistaltic contractions
-
Antral nipple sign [43] (ie, a prolapse of redundant mucosa into the antrum, which creates a pseudomass) (see the image below)
-
Other findings include reversible portal venous gas; nonuniform echogenicity of the pyloric muscle
Degree of confidence
A positive hypertrophic pyloric stenosis finding by ultrasonography almost always indicates this condition is present. A negative examination can be false in a patient who is seen early in the disease or in a younger patient whose MT is less than 3 mm.
Many articles since Teele and Smith’s original description of the ultrasound diagnosis of HPS have justified measurement criteria based on each group’s specificity, sensitivity, and positive predictive value. Values reported in the literature range from an MT of 2.5 to 4 mm or thicker and a CL of 14 to 19 mm. The risk of a false-positive test may result in a negative laparotomy. Forman concluded that an MT of 4 mm and a CL of 16-20 mm may decrease sensitivity for HPS, but these thresholds avoid a negative laparotomy. [46] The authors commented that a patient with a normal ultrasound, based on these criteria, and persistent symptoms may be reevaluated. Of note, as discussed above, younger infants may have relatively smaller measurements of the pylorus in the setting of HPS.
False positives/negatives
The diagnostic accuracy of ultrasonography for hypertrophic pyloric stenosis is high. Sensitivity and specificity approach 100%. [27, 28] Possible sources of false negatives are an overdistended stomach (the pylorus is hidden behind the antrum), failure to identify the pylorus (eg, operator inexperience in performing ultrasonography for evaluation of this condition), and a small infant or early presentation.
Iqbal et al showed that age and weight correlate positively with ultrasound measurement of the pylorus in pyloric stenosis and that it did not affect the diagnostic criteria. In their series of 318 ultrasound studies, there was 100% sensitivity and 100% specificity for HPS when either the pyloric channel thickness or length of 3 mm and 15 mm, respectively, were met. Further, they demonstrated a negative correlation between pyloric measurement, age, and weight when the pylorus was normal. [47] If a question remains, follow-up ultrasound can be performed in a few days to reassess muscle thickness.
Another possible source for false-positive findings is pylorospasm (typically transient). In this scenario, one should wait up to 15 minutes and remeasure the muscle to avoid mistaking muscle spasm for hypertrophy.
Although a false-negative clinical diagnosis causes diagnostic delay, a false-positive diagnosis results in a negative laparotomy. Therefore, imaging has become more important in the diagnosis of hypertrophic pyloric stenosis.
-
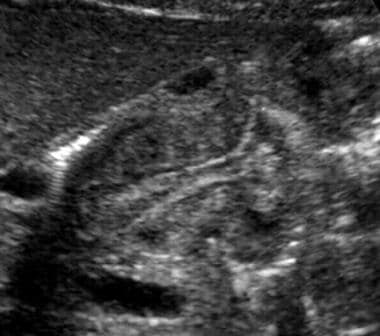
Longitudinal ultrasonogram of the pylorus in a patient with surgically proven hypertrophic pyloric stenosis. Note the thickened, circular muscle, elongated pylorus, and narrowed pyloric channel.
-
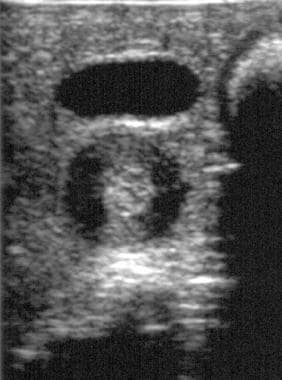
Transverse ultrasonographic image in a patient with proven hypertrophic pyloric stenosis demonstrates the target sign and heterogeneous echo texture of the muscular layer (the pylorus is deep to the anechoic gallbladder).
-
Longitudinal ultrasonogram in a patient with hypertrophic pyloric stenosis demonstrates a redundant mucosa that creates the antral nipple sign.
-
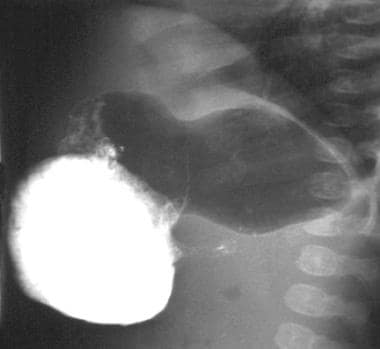
Lateral view from an upper gastrointestinal study demonstrates the double-track sign.
-
Upper gastrointestinal study from a child shows the string sign (see inset).
-
Supine radiograph in an infant with vomiting demonstrates the caterpillar sign of active gastric hyperperistalsis.